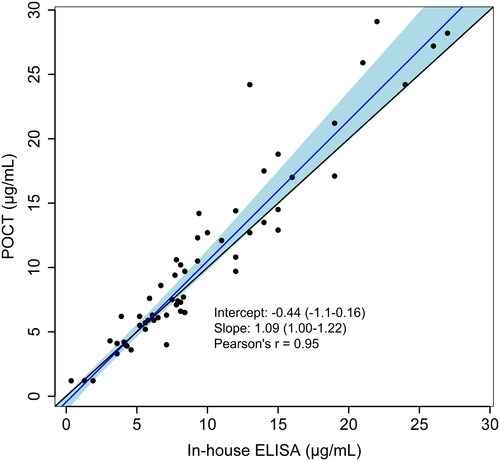
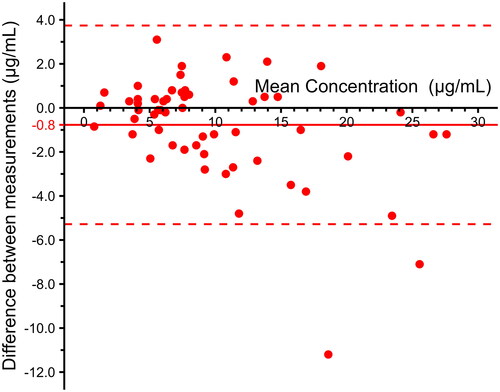
Figures & data
Table 1. Demographics and clinical characteristics of patients with inflammatory bowel disease receiving infliximab treatment (n = 61).
Supplemental Material
Download Zip (370.2 KB)Data availability statement
No additional data are available due to the Swedish data protection regulation.