Figures & data
Figure 1. Screenee flowchart. Some people were assigned to a group but could not be invited because they had died, emigrated, or could not be located at a valid address. PCOL group = primary colonoscopy group, FIT group = faecal immunochemical testing group.
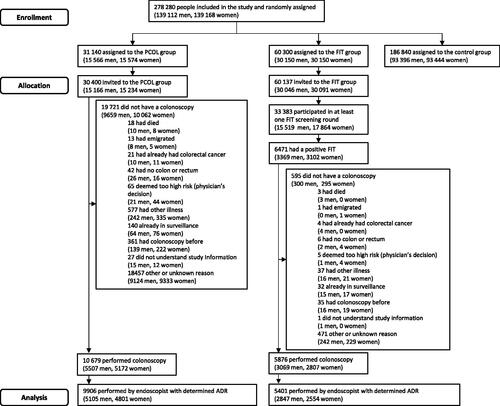
Table 1. Screenee and practitioner characteristics for SCREESCO study colonoscopies, stratified by study group (PCOL group = primary colonoscopy group, FIT group = faecal immunochemical testing group), endoscopist ADR (high ADR = adenoma detection rate is at least 20% in the primary colonoscopy group, at least 35% in the faecal immunochemical testing group), and screenee sex.
Figure 2. Forest plot of adjusted risk ratios (RR) of men vs women (reference) for each outcome for screenees in the PCOL group stratified by endoscopist ADR (low = blue, high = red). analyses were adjusted for calendar period of colonoscopy. The p-value from the multiplicative interaction test of endoscopist skill and sex is colored black. PCOL group = primary colonoscopy group.
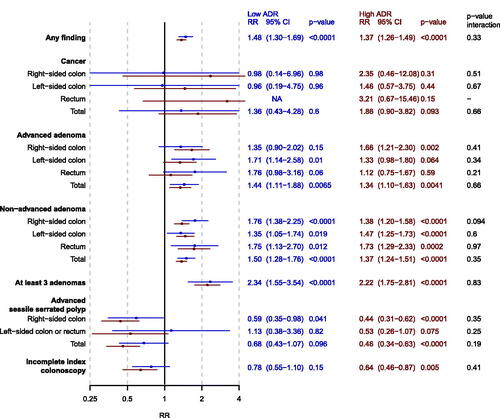
Table 2. Yield by sex, study group and endoscopist ADR. PCOL group = primary colonoscopy group, FIT group = faecal immunochemical testing group.
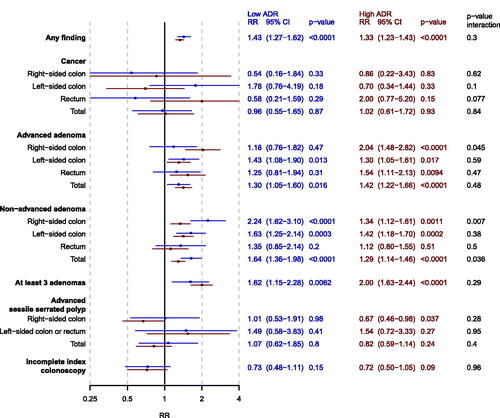
Figure 3. Forest plot of adjusted risk ratios (RR) of men vs women (reference) for each outcome for screenees in the FIT group stratified by endoscopist ADR (low = blue, high = red). analyses were adjusted for calendar period of colonoscopy. The p-value from the multiplicative interaction test of endoscopist ADR and sex is colored black. PCOL group = primary colonoscopy group.
Supplemental Material
Download MS Word (17.2 KB)Supplemental Material
Download MS Word (11.9 KB)Supplemental Material
Download PDF (17.4 KB)Supplemental Material
Download PDF (31.2 KB)Supplemental Material
Download PDF (191 KB)Data availability statement
For data sharing questions, please contact the study principal investigator at [email protected]. The study protocol and statistical analysis plan are available on request. Deidentified individual participant data that underlie the results reported in this Article (including in the appendix), can be made available to researchers after request to the SCREESCO Steering Committee. Researchers must provide a methodologically sound proposal for a project that conforms with the Swedish Ethical Review Authority permit for the project and will need to sign a data access agreement. Data will be made available at a secure remote server to achieve the aims in the approved proposal. Data will be available from 3 months after publication and until 3 years after publication of the Article. Proposals regarding the data underlying this Article may be submitted up to 2 years after publication. The SCREESCO study will not carry the costs of external projects.
