Figures & data
Table 1 Selected chemical and physical properties of Tateyama soil (Cambisol), peat moss (PM), and sawdust (SD) compost served for the cultivation experiment and soils amended with PM and SD compost plus ferrous sulfate. The values for PM and SD compost amended soils were measured before planting blueberry seedlings in April 2008
Table 2 Total dry weight (shoot and root) of blueberry plants, annual blueberry yields, and total nitrogen (N) uptake of blueberry plant over the experimental period in each treatment
Figure 1 Soil pH changes during the experimental period as affected by organic amendments. AS, ammonium sulfate applied alone; AS + PM, ammonium sulfate applied with peat moss; AS + SD, ammonium sulfate applied with sawdust compost and ferrous sulfate. Bars show least significant difference (LSD) (α = 0.05). Vertical arrows show the timing of nitrogen fertilizer applications.
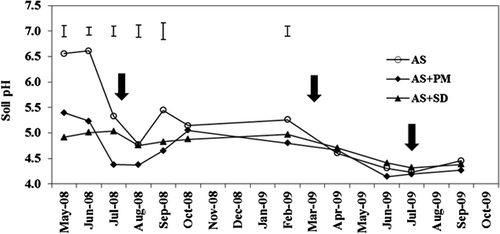
Figure 2 Changes in ammonium ion () (a) and nitrate ion (
) (b) concentrations in soils during the experimental period. AS, ammonium sulfate applied alone; AS + PM, ammonium sulfate applied with peat moss; AS + SD, ammonium sulfate applied with sawdust compost and ferrous sulfate. Bars show least significant difference (LSD) (α = 0.05). Vertical arrows show the timing of nitrogen fertilizer applications.
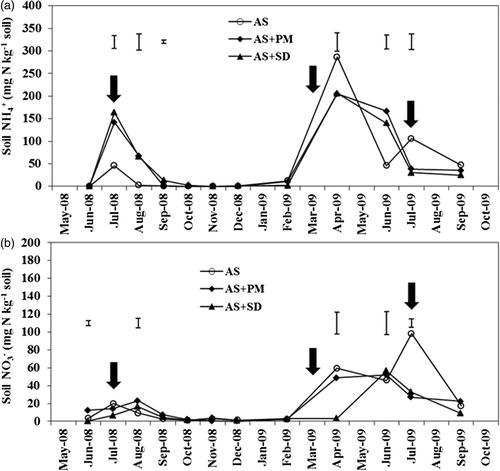
Figure 3 Changes in daily nitrous oxide (N2O) fluxes (a), precipitation (b), and soil temperature at 5-cm depth (c) in 2008 and 2009. AS, ammonium sulfate applied alone; AS + PM, ammonium sulfate applied with peat moss; AS + SD, ammonium sulfate applied with sawdust compost and ferrous sulfate. Bars show least significant difference (LSD) (α = 0.05). Vertical arrows show the timing of nitrogen fertilizer applications.
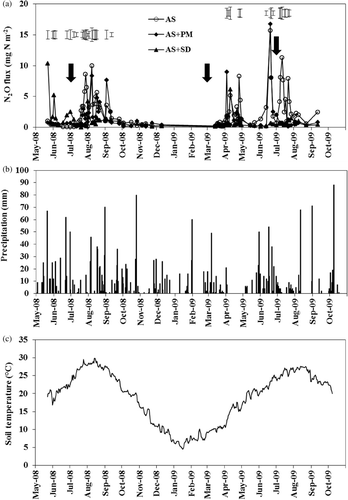
Table 3 Total mineral nitrogen (N) applied to blueberry cultivating soils, cumulative N loss as emitted nitrous oxide (N2O), and emission factors of N2O throughout the experimental period in each treatment
Figure 4 Cumulative nitrogen (N) losses as ammonium ion () and nitrate ion (
) in leachates in 2008 (a) and 2009 (b). AS, ammonium sulfate applied alone; AS + PM, ammonium sulfate applied with peat moss; AS + SD, ammonium sulfate applied with sawdust compost and ferrous sulfate. Vertical arrows show the timing of nitrogen fertilizer applications.
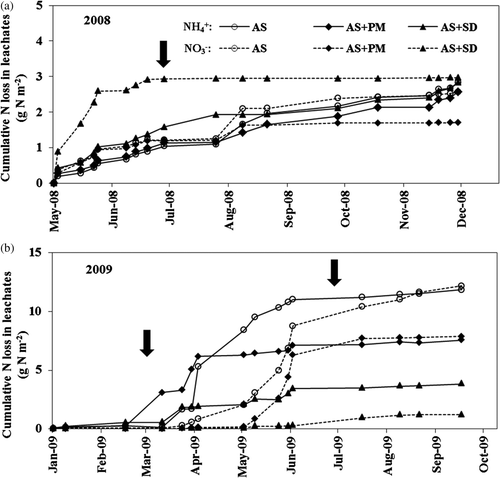
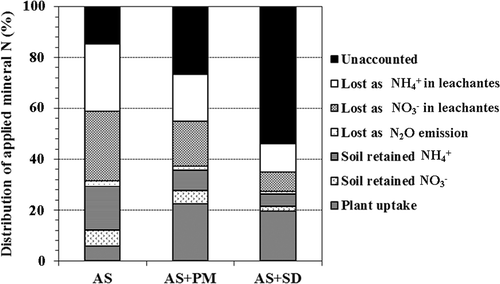
Figure 5 Distribution of mineral nitrogen (N) applied as ammonium sulfate after two-year cultivation of blueberry. AS, ammonium sulfate alone; AS + PM, ammonium sulfate with peat moss; AS + SD, ammonium sulfate with sawdust compost and ferrous sulfate. N2O, nitrous oxide; , ammonium ion;
, nitrate ion.