Figures & data
Figure 1 Phylogenetic and expression analyses of NtSUT1-L and S cloned from cultured tobacco (Nicotiana tabacum L.) cell line SL. In (A), phylogenetic analysis of the newly isolated NtSUT1 genes (L, S) was performed together with the previously reported NtSUT’s. Potato StSUT1 and StSUT4 were used as an outgroup for phylogenetic analysis. Accession numbers of sucrose transporter amino acid sequences are as follows: NtSUT1-L (AB823663), NtSUT1-S (AB823664), NtSUT1a (X82276), NtSUT1x (AM491605), NtSUT1y (FM164639), NtSUT3 (AAD34610), NtSUT4 (BAI60050), StSUT1 (X69165), StSUT4 (AF237780). In (B), the expression levels of NtSUT1-L and NtSUT1-S were examined in tobacco cell lines (SL, BY-2) at the logarithmic phase of growth. Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed, using primer sets specific for NtSUT1-L (identical to NtSUTa) or NtSUT-S (identical to NtSUTy), respectively. Relative expression levels were normalized against the values of the Actin9 transcripts. Each value represents the mean ± standard error (SE) of three samples from three independent experiments. For each cell line, significant differences between genes are indicated with different lower case letters, which were determined by least significant difference (LSD) test at P < 0.05.
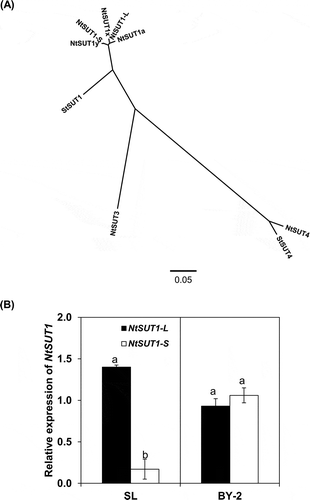
Figure 2 Characterization of OX and RNAi lines of NtSUT1 in BY-2. In (A), expression analyses of NtSUT1 were performed in BY-2 cell lines (wild type (WT), over-expressor (OX)-1, OX-2, RNAi-1) during the logarithmic phase of growth in nutrient medium. NtSUT1 expression levels were evaluated by real-time reverse transcription-polymerase chain reaction (RT-PCR) by using specific primer sets for the NtSUT1-L (WT, OXs) and the 3’-UTR conserved region of NtSUT1 (WT, RNAi), respectively. Relative expression levels were normalized against the values of the Actin9 transcripts. Each value represents the mean ± standard error (SE) of three samples from three independent experiments. For each analysis, significant differences among lines are indicated with different lower case letters, which were determined by least significant difference (LSD) test at P < 0.05. In (B), sucrose uptake as determined in a nutrient medium. Cells were suspended in a nutrient medium at 50 mg fresh weight (FW) per mL and sucrose uptake was monitored at the times indicated after the addition of 14C-sucrose at 0 h, as described in the Materials and methods section. The uptake values are shown per cells at one mg FW, based on the FW of the culture determined at 0 time. Each value represents the average value from two independent experiments.
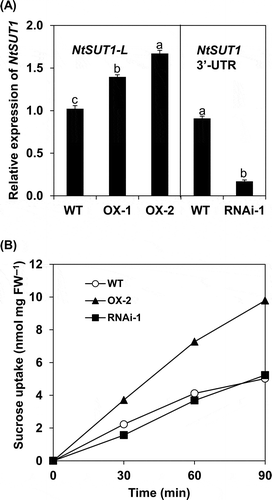
Figure 3 Soluble sugar content and mass doubling time during logarithmic phase of growth in BY-2 cell lines (wild type (WT), over-expressor (OX)-1, OX-2, RNAi-1). Soluble sugar content (A) and mass doubling time (B) were determined in each cell line as described in the Materials and methods section. Each value represents the mean ± standard error (SE) of three independent experiments. In (A), significant differences among lines are indicated with different lower case letters, which were determined by least significant difference (LSD) test at P < 0.05. Data in (B) are not statistically different. In (C), soluble sugar content (Y) and mass doubling time (X) of each line is plotted. The relationship between these values can be shown as Y = –1.0831X+51.206 (R2 = 0.9818).
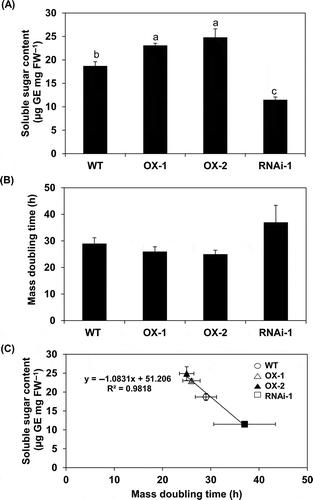
Figure 4 Effects of 2,4-dichlorophenoxyacetic acid (2,4-D) and aluminum (Al) on NtSUT1-L expression in BY-2 cell lines (wild type (WT), over-expressor (OX)-2). Cells at the logarithmic phase of growth were treated without (control) or with Al (50 µM) in the absence or presence of 2,4-D (1.5 µM) for 18 h. The NtSUT1 expression levels of the cells were evaluated by real-time reverse transcription polymerase chain reaction (RT PCR) analysis using specific primers for NtSUT1-L. Relative expression levels were normalized against the values of the Actin9 transcripts. Each value represents the mean ± standard error (SE) of three samples from three independent experiments. For each treatment condition, significant differences between lines are indicated with different lower case letters, which were determined by least significant difference (LSD) test at P < 0.05.
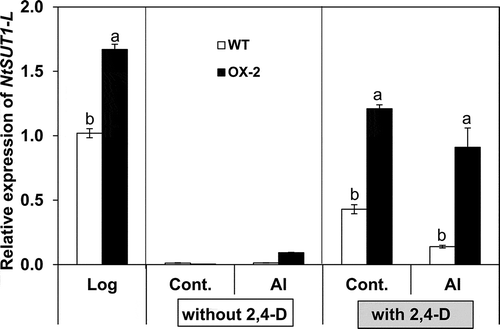
Figure 5 Effects of aluminum (Al) on sucrose uptake rate and soluble sugar content during Al treatment in BY-2 cell lines (wild type (WT), over-expressor (OX)-2, RNAi-1). Cells at the logarithmic phase of growth were suspended in Al treatment medium at 10 mg fresh weight (FW) mL–1 (initiation) then incubated without (control) or with Al (50 µM) in the presence or absence of 2,4-dichlorophenoxyacetic acid (2,4-D) (1.5 µM). In (A), sucrose uptake was monitored only in the presence of 2,4-D for up to 6 h after the addition of 14C-sucrose at 0 h. The uptake value was shown per cells contained in one mL aliquot of the culture. Each value represents the average value from two independent experiments. In (B), soluble sugar contents were determined after 18 h treatment in the absence or presence of 2,4-D as described in the Materials and methods section and are shown per cells contained in one mL aliquot of the culture. Each value represents the mean ± standard error (SE) of three samples from three independent experiments. For each treatment, significant differences among lines are indicated with different lower case letters, which were determined by least significant difference (LSD) test at P < 0.05.
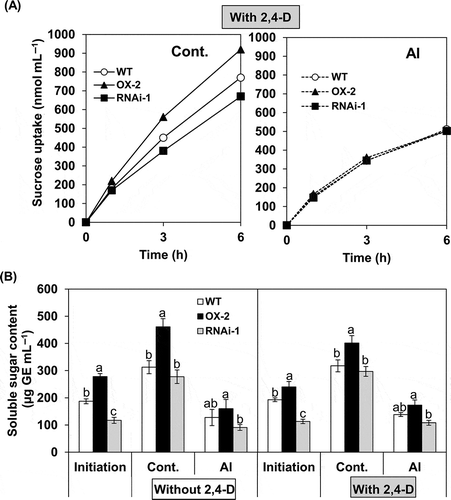
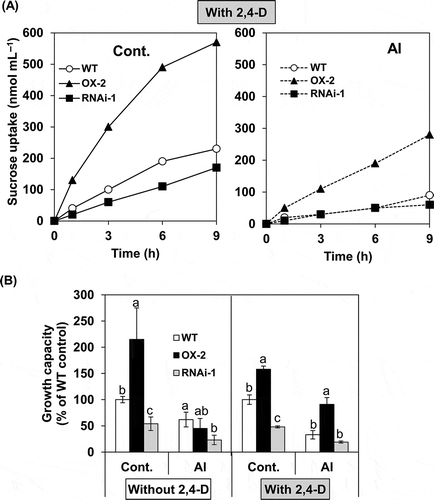
Figure 6 Effects of aluminum (Al) on sucrose uptake rate and growth capacity during post-Al treatment culture in BY-2 cell lines (wild type (WT), over-expressor (OX)-2, RNAi-1). After Al treatment in the absence or presence of 2,4-dichlorophenoxyacetic acid (2,4-D) (1.5 µM), cells were transferred into fresh nutrient medium and cultured under standard conditions. In (A), sucrose uptake during post-Al treatment culture was monitored only in the cells which had been treated without or with Al in the presence of 2,4-D. 14C-sucrose was added at 24 h of the post-treatment culture and cellular radioactivity was determined at indicated times. The uptake value was shown per cells contained in one mL aliquot of the culture. Each value represents the average value from two independent experiments. In (B), growth capacity was determined in the cells which had been treated without or with Al in the absence or presence of 2,4-D, and then cultured for 6 d (for the cells treated without 2,4-D) or 4 d (for the cells treated with 2,4-D), as described in the Materials and methods section. Each value represents the mean ± standard error (SE) of three samples from three independent experiments. For each treatment, significant differences among lines are indicated with different lower case letters, which were determined by least significant difference (LSD) test at P < 0.05.