Figures & data
Table 1 Sequences of primers and probe used for qRT-PCR analysis
Figure 1 Changes in carbohydrate content (% dry weight) in tobacco (Nicotiana tabacum L.) leaves during the curing process. Starch (open circles), total main sugars (glucose, fructose and sucrose; closed circles), and total carbohydrates (open triangles). Vertical bars, standard error (SE) (n = 6).
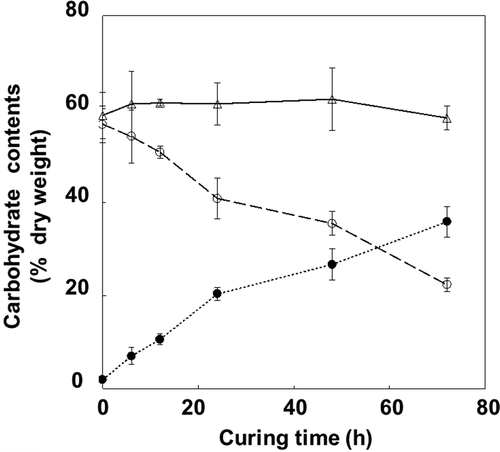
Figure 2 Changes in water content levels in tobacco (Nicotiana tabacum L.) leaves during the curing process. Data were expressed to 100 at harvesting (time point 0). Leaves just after harvest contained 9-fold water content relative to dry materials. Vertical bars, standard error (SE) (n = 6).
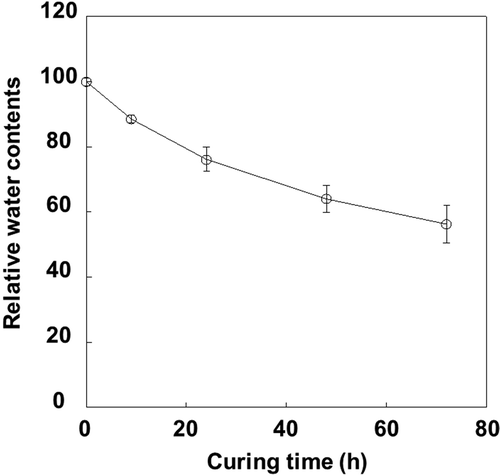
Figure 3 Changes in major (a) and minor (b) tobacco (Nicotiana tabacum L.) leaf sugar contents (% dry weight) during the curing process. (a) Glucose (open circles), fructose (closed circles) and sucrose (open triangles). (b) Raffinose (open circles), galactose (closed circles), polyglucan (open triangles) and maltose (closed triangles). Vertical bars, standard error (SE) (n = 4).
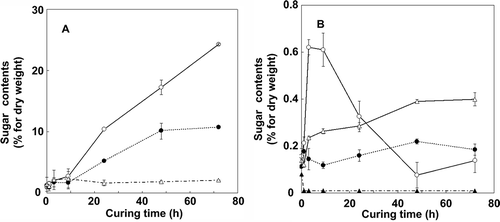
Figure 4 Changes in α-amylase, β-amylase, starch phosphorylase, and isoamylase activities in tobacco (Nicotiana tabacum L.) leaves during the curing process. (a) α-amylase (open circles), β-amylase (closed circles) and starch phosphorylase (open triangles). (b) Isoamylase (closed triangles). All enzyme activities were measured at 25°C. Vertical bars, standard error (SE) (n = 4).
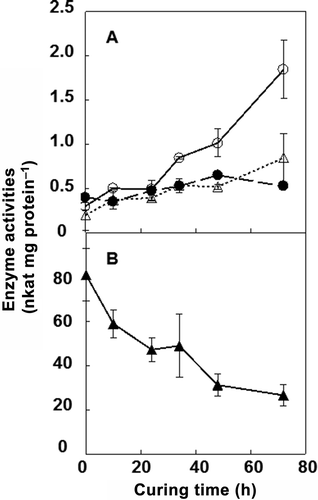
Figure 5 Immunoblot analysis of α-amylase1 protein in tobacco (Nicotiana tabacum L.) leaves during the curing process. Zero curing time indicated at harvesting. The other time points indicated at curing time after start. The arrow indicates α-amylase1 protein at MW 46.6 kDa.
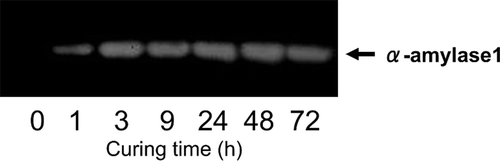
Figure 6 Changes in transcript levels of α-amylase (a) and other starch degradation enzyme genes (b) and (c) in tobacco (Nicotiana tabacum L.) leaves during the curing process. (a) NtAMY1 (open circles), NtAMY2 (closed circles), NtAMY3 (open triangles) and NtAMY4 (closed triangles). (b) NtBAM1 (open circles), NtBAM2 (closed circles), NtISO1 (open triangles) and NtISO2 (closed triangles). (c) NtGWD1(open squares) and NtGWD3 (closed squares). Relative levels were analyzed by comparative threshold cycle (CT) using the β-actin gene (NtACT2). Transcript levels were set to 1 at harvesting (time point 0) for all genes. Vertical bars, standard error (SE) (n = 6).
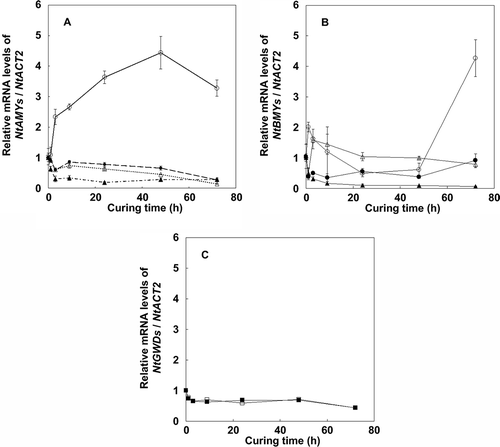
Figure 7 Changes in transcript levels of individual NtAMYs in leaves attached to tobacco (Nicotiana tabacum L.) whole plants under various stress conditions: NtAMY1 (closed bars), NtAMY2 (stripe bars), NtAMY3 (dot bars) and NtAMY4 (opened bars). Whole plants were kept in a temperature and humidity chamber in darkness at 25°C and 82% relative humidity (RH) for 24 h. Injury stress was applied by topping under the same conditions. High-temperature stress was applied at 42°C, whereas osmotic stress was applied by subjecting the plants to 20% (w/v) polyethylene glycol 6000 under the same conditions. Each transcript level was normalized to that of the β-actin gene (NtACT2), and shown as a relative value to control. Different letters designate statistically different values for each treatment [analysis of variance (ANOVA) with Tukey’s highly significant difference (HSD) test, P < 0.05]. Vertical bars, standard error (SE) (n = 6).
![Figure 7 Changes in transcript levels of individual NtAMYs in leaves attached to tobacco (Nicotiana tabacum L.) whole plants under various stress conditions: NtAMY1 (closed bars), NtAMY2 (stripe bars), NtAMY3 (dot bars) and NtAMY4 (opened bars). Whole plants were kept in a temperature and humidity chamber in darkness at 25°C and 82% relative humidity (RH) for 24 h. Injury stress was applied by topping under the same conditions. High-temperature stress was applied at 42°C, whereas osmotic stress was applied by subjecting the plants to 20% (w/v) polyethylene glycol 6000 under the same conditions. Each transcript level was normalized to that of the β-actin gene (NtACT2), and shown as a relative value to control. Different letters designate statistically different values for each treatment [analysis of variance (ANOVA) with Tukey’s highly significant difference (HSD) test, P < 0.05]. Vertical bars, standard error (SE) (n = 6).](/cms/asset/c21cf968-4957-46ca-b04a-99eff40759f9/tssp_a_842884_f0007_oc.jpg)
Figure 8 Total sugar content (glucose, fructose, and sucrose) of leaves attached to tobacco (Nicotiana tabacum L.) whole plants under the same stress conditions as in . As an additional treatment in , curing was applied to detached in darkness at 42°C and 82% relative humidity (RH) for 24 h. Different letters designate statistically different values for each treatment [analysis of variance (ANOVA) with Tukey’s highly significant difference (HSD) test, P < 0.05]. Vertical bars, standard error (SE) (n = 4).
![Figure 8 Total sugar content (glucose, fructose, and sucrose) of leaves attached to tobacco (Nicotiana tabacum L.) whole plants under the same stress conditions as in Fig. 7. As an additional treatment in Fig. 7, curing was applied to detached in darkness at 42°C and 82% relative humidity (RH) for 24 h. Different letters designate statistically different values for each treatment [analysis of variance (ANOVA) with Tukey’s highly significant difference (HSD) test, P < 0.05]. Vertical bars, standard error (SE) (n = 4).](/cms/asset/89d7e1be-7f77-44ab-8150-19fe050058af/tssp_a_842884_f0008_b.gif)