Figures & data
Table 1. Morphological and physico-chemical properties of soil.
Figure 1. (a) Scanning electron microscope image of siderite. (b) Energy-dispersive X-ray (EDX) mapping image of iron in siderite. (c) EDX spectrum corresponding to point 1 in a; O: oxygen, Al: aluminum, Si: silicon, P: phosphorus, Ca: calcium, Mn: manganese, Fe: iron. (d) d1: X-ray diffraction (XRD) chart of powdered siderite sample; a rising baseline due to an iron-rich sample was corrected to be nearly flat to compare the peak positions with reported data. d2: XRD chart of powdered siderite reported by Brindley and Brown (Citation1980), Q: quartz.
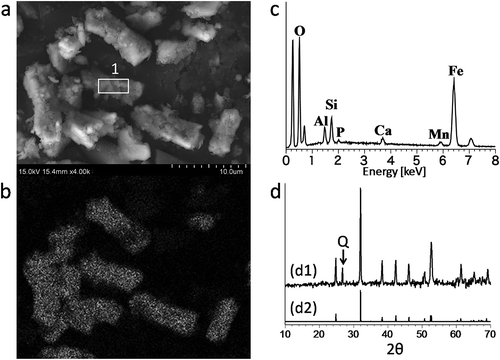
Table 2. Aqua regia digestible and 2.88 mol L−1 hydrochloric acid (10% HCl) soluble element concentrations of soil, ratio of 10% HCl soluble concentration to aqua regia digestible concentration (HCl /Aqua regia), dissolution rate of soil in 10% HCl.
Table 3. Element concentrations and dissolution rate corresponding to siderite size dissolved in 2.88 mol L−1 hydrochloric acid (10% HCl), and element concentration ratio of siderite to soil.
Figure 2. R0 values of elements corresponding to siderite size (< 1 cm, 1–2 cm and > 2 cm). Mn, Fe, P, Ca, Co, Cr, Mo, Y, Si, Sc, Sr, Ba, Sn, As, K, Al, Zn, S, Na, Mg, Li, Cu, Sb, Pb, and Cd are manganese, iron, phosphorus, calcium, cobalt, chromium, molybdenum, yttrium, silicon, scandium, strontium, barium, tin, arsenic, potassium, aluminum, zinc, sulfur, sodium, magnesium, lithium, copper, antimony, lead, and cadmium, respectively. R0 value is nondimensional parameter calculated by (Csiderite/Csoil) = R0(Tisiderite/Tisoil). Csoil and Csiderite are element concentrations of soil and siderite, and Tisoil and Tisiderite are Ti concentrations of soil and siderite, respectively. Element concentrations of soil were the average of Cr2 and Cr3 horizons. R0 values are arranged from highest to lowest. Vertical bar represents standard deviations of the means (n = 2). P values of the effect of siderite particle size on the R0 value of all elements were > 0.05 (one-way analysis of variance, ANOVA).
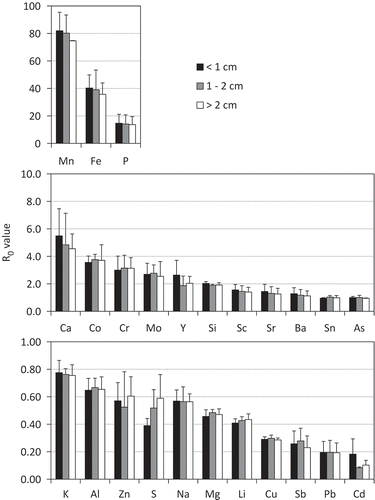
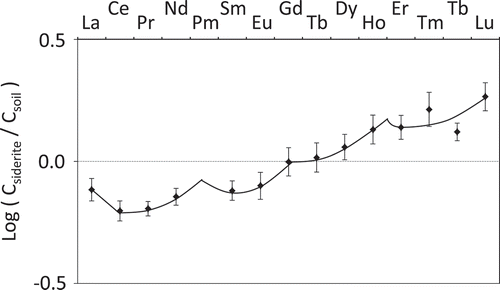
Figure 3. Rare earth element (REE) distribution pattern of siderite–soil system.
CSiderite: 2.88 mol L−1 hydrochloric acid (HCl) soluble element concentration of siderite, Csoil: 2.88 mol L−1 HCl soluble element concentration of soil. La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Tb, and Lu are lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium, respectively. Promethium of atomic number 61 does not exist in nature because Pm does not have a stable isotope. The curve between Tm and Tb was passed through their intermediate point. Vertical bar represents ranges of values (n = 2).