Figures & data
Table 1. Some chemical properties of the two soils used.
Figure 1. Silicate and phosphate adsorption isotherms at pH 5.0, 5.5, 6.0 and 6.5 for the two study soils. Standard errors of the mean shown.
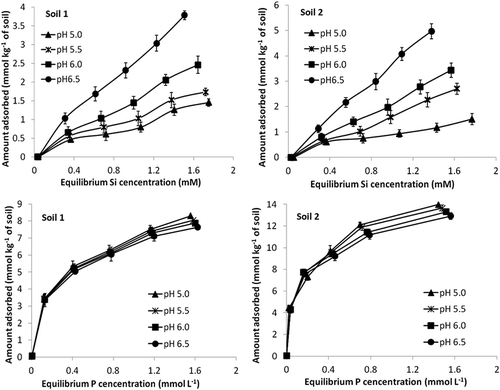
Figure 2. Effect of pH on the concentration of Si and P in equilibrium solutions after addition of Si and/or P at an initial concentration of 1.6 mM. Equilibrium was with either monoelement (Si or P) or multielement (Si + P) solutions. Standard errors of the mean shown.
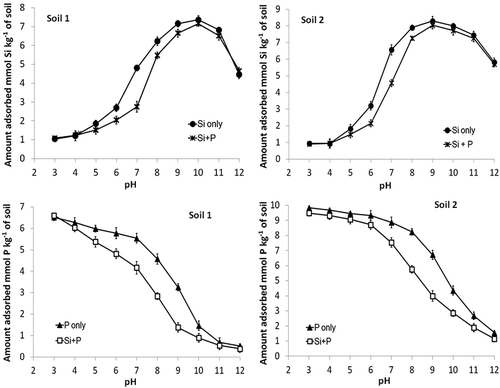
Table 2. Langmuir and Freundlich isotherm constants and coefficients of determination (R2) for adsorption of Si and P onto the soils at pH 5.0, 5.5, 6.0 and 6.5.
Figure 3. Concentrations of resin-extractable P and P adsorption isotherms for soil 1 treated with three rates of Si (R1, R2 and R3 = 300, 600 and 1200 mg Si kg−1 respectively) as CaSiO3 which gave soil pH values of 5.4, 5.6 and 6.5 or an appropriate amount of Ca(OH)2 to give the same pH values. Standard errors of the mean for values on adsorption isotherm shown. Values of extractable P followed by the same letter are not significantly different P ≤ .05.
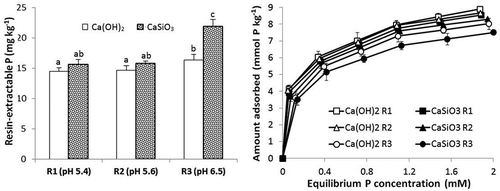
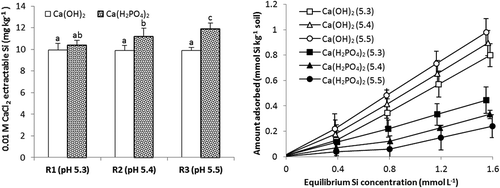
Figure 4. Concentrations of CaCl2-extractable Si and Si adsorption isotherms for soil 1 treated with three rates of P (R1, R2 and R3 = 30, 60 and 100 mg P kg−1 respectively) as Ca(H2PO4)2 which gave soil pH values of 5.3, 5.4 and 5.5 or an appropriate amount of Ca(OH)2 to give the same pH values. Standard errors of the mean for values on adsorption isotherm shown. Values of extractable Si followed by the same letter are not significantly different P ≤ .05.