Figures & data
Figure 1. Amount of outer sphere complexed cations in Tongliao soil with composts mixed at various ratios. Dotted line indicates the initial contents in the soil. Error bars indicate standard deviation (n = 3)
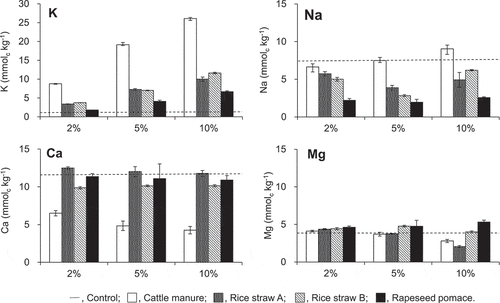
Table 1. Properties of the compost samples used
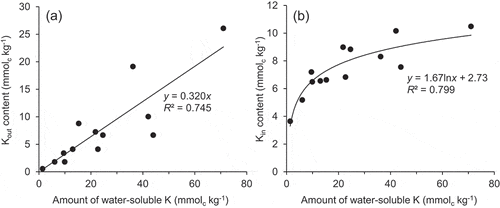
Table 2. Contents of water-soluble basic cations and basic cations forming outer sphere and inner sphere complex in Tongliao soil
Figure 2. Amount of inner sphere complexed cations in the Tongliao soil with composts mixed at various ratios. Dotted line indicates the initial contents in the soil. Error bars indicate standard deviation (n = 3)
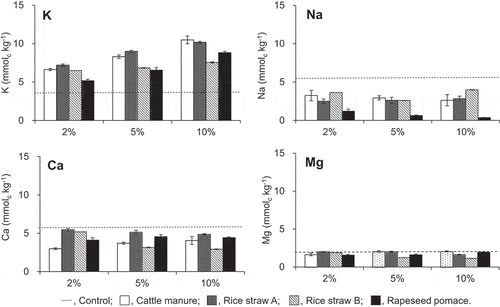
Figure 3. Relationship between the mixing ratio of the compost and soil pH. Error bars indicate standard deviation (n = 3)
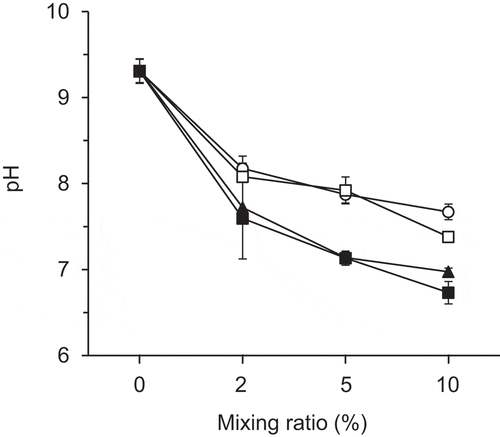
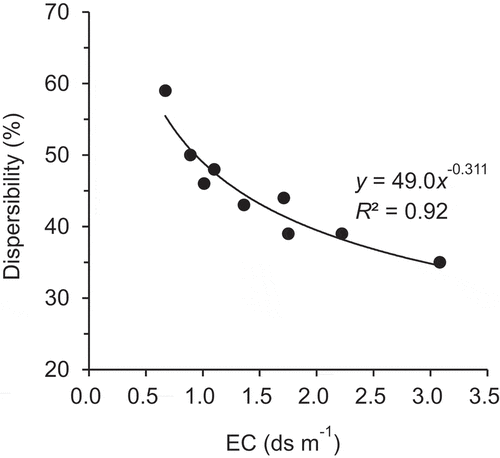
Table 3. Dispersibility, electrical conductivity (EC), exchangeable potassium percentage (EPP), and exchangeable sodium percentage (ESP) of Tongliao soil with compost mixed at various ratios
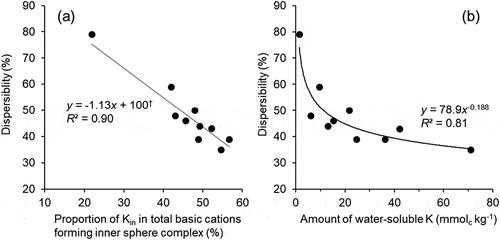
Figure 6. Relationship between relative abundance of Kin in total cations forming the inner sphere complex and dispersibility† (a) and that between amount of water-soluble K and dispersibility (b).
†Regression equation when the data from control soil (dispersibility, 79%) was excluded is: y = -1.13x +100 (R² = 0.67; P < 0.01)