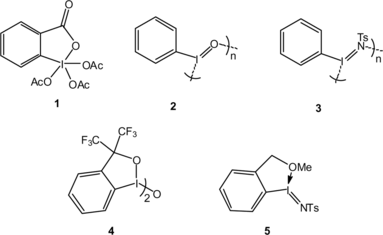
Figures & data
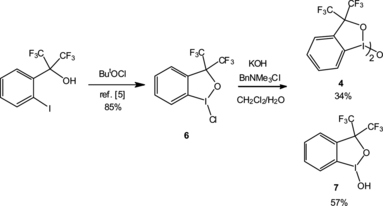
Figure 1 (a) Molecular structure of 6, left-hand side. Selected bond distances and angles: I–C(1) 2.108 (2), I–O 2.109 (6), I–Cl 2.448 (14) Å; Cl–I–O 171.63 (4), C(1)–I–Cl 92.60 (6), C(1)–I–O 79.24 (7)°. (b) Molecular structure of 4, right-hand side. Selected bond distances and angles: I(1)–O(2) 2.0296 (15), I(1)–C(1) 2.097 (2), I(1)–O(1) 2.1548 (15), O(2)–I (1A) 2.0300 (15), I(1A)–C(1A) 2.105 (2), I(1A)-O(1A), 2.1407 (16) Å; O(2)–I(1)–O(1) 167.72 (6), O(2)–I(1A)–O(1A) 168.00 (6)°.
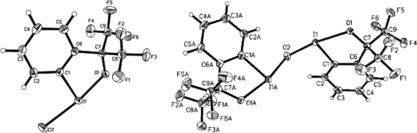
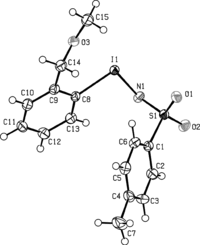
Figure 2 Selected bond distances and angles for (5): C(8)–I(1) 2.13, O(3)–I(1) 2.65 Å; N(1)–I(1)–C(8) 98.48°. Data averaged from two independent molecules.