Figures & data
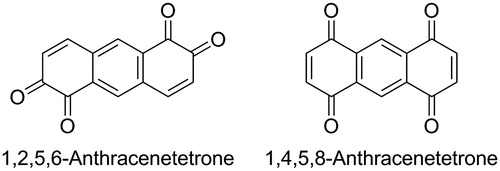
Scheme 1. Facile synthesis of 1,4,9,10-antracenetrone starting from 1,4-dihydroxy-9,10-anthraquinone (top), molecular structure of 1,2,5,6- and 1,4,5,8-anthracenetetrone (bottom).
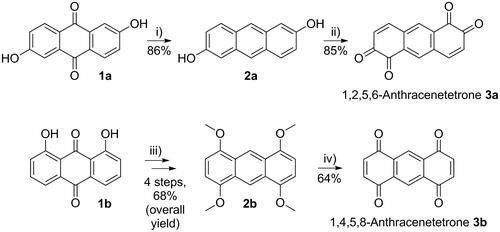
Scheme 2. (i) Reduction of 2,6-dihydroxy-9,10-anthraquinone (1a) to 2,6-dihydroxyanthracene (2a). Conditions: NaBH4, 1 M Na2CO3 solution, under argon, RT, overnight. (ii) Oxidation to 1,2,5,6-anthracenetetrone (3a). Conditions: benzeneseleninic acid anhydride, dry THF, under argon, 50 °C, 3 h. (iii) Conversion of 1,8-dihydroxy-9,10-anthraquinone (1b) into 1,4,5,8-tetramethoxyanthracene (2b). Conditions: see Ref. [Citation28], and (iv) Oxidation to 1,4,5,8-anthracenetetrone 3b. Conditions: silica-supported ceric ammonium nitrate (CAN), CH2Cl2, RT, 1 h.
![Scheme 2. (i) Reduction of 2,6-dihydroxy-9,10-anthraquinone (1a) to 2,6-dihydroxyanthracene (2a). Conditions: NaBH4, 1 M Na2CO3 solution, under argon, RT, overnight. (ii) Oxidation to 1,2,5,6-anthracenetetrone (3a). Conditions: benzeneseleninic acid anhydride, dry THF, under argon, 50 °C, 3 h. (iii) Conversion of 1,8-dihydroxy-9,10-anthraquinone (1b) into 1,4,5,8-tetramethoxyanthracene (2b). Conditions: see Ref. [Citation28], and (iv) Oxidation to 1,4,5,8-anthracenetetrone 3b. Conditions: silica-supported ceric ammonium nitrate (CAN), CH2Cl2, RT, 1 h.](/cms/asset/cbe3ab5e-6ad3-47d7-8ce2-393f70798ac3/lsyc_a_1483027_sch0002_b.jpg)
Figure 1. Crystal structure of 3b·1,4-dioxane viewed down (a) [100] and (b) [010]. (c) The molecular structure of 3b. C (grey) and O (red) atoms are represented by ellipsoids drawn at the 50% probability levels; H atoms by white spheres of arbitrary radius.
![Figure 1. Crystal structure of 3b·1,4-dioxane viewed down (a) [100] and (b) [010]. (c) The molecular structure of 3b. C (grey) and O (red) atoms are represented by ellipsoids drawn at the 50% probability levels; H atoms by white spheres of arbitrary radius.](/cms/asset/5d68e1f9-605f-4cca-addd-7f48a2892f52/lsyc_a_1483027_f0001_c.jpg)
