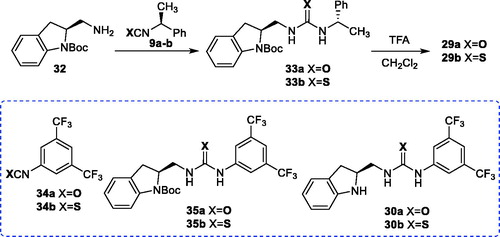
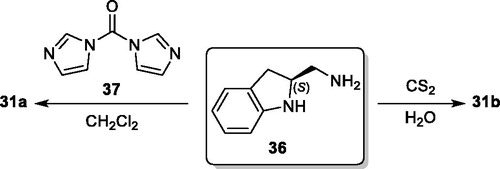
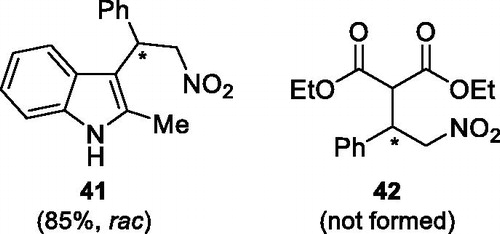
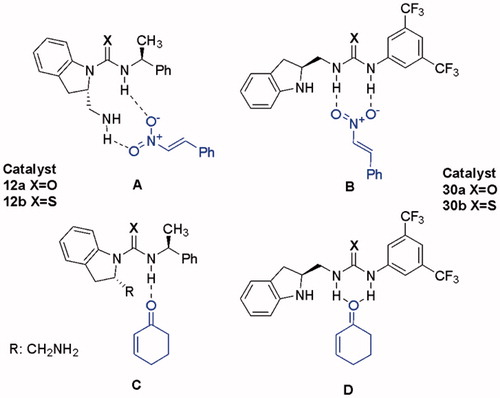
Figures & data
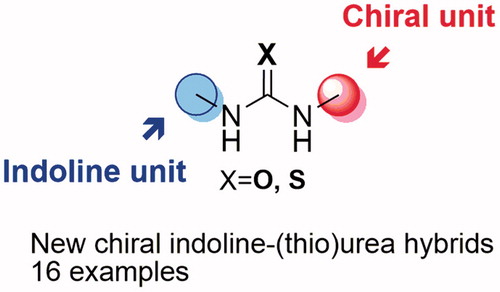
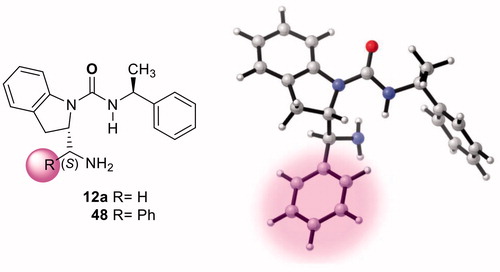
Table 1. Catalyst screening for the asymmetric Michael addition of indole (38) to β-nitrostyrene (39).a
Table 2. Catalyst screening for the asymmetric Morita–Baylis–Hillman (MBH) reaction of 2-cyclohexen-1-one (43) with benzaldehydes (44 and 45).a
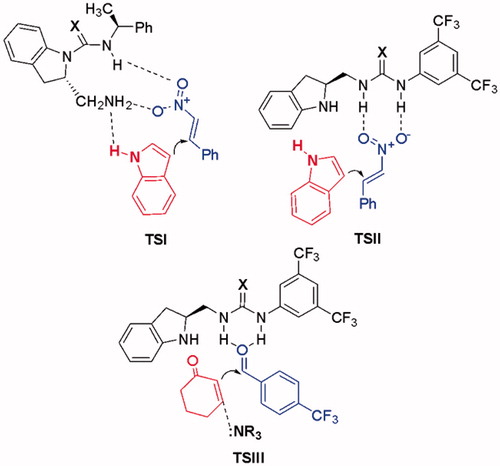
Figure 7. Free energy profile for 12a and 30a catalyzed and uncatalyzed Michael addition reaction of indole (38) to β-nitrostyrene (39). M06-2X/6-31 + G(d,p) with IEF-PCM in toluene; free energies in kcal/mol at 298 K and 1 atm. Results for TS-S, TS-12a-S and TS-30a-S are shown in parenthesis.
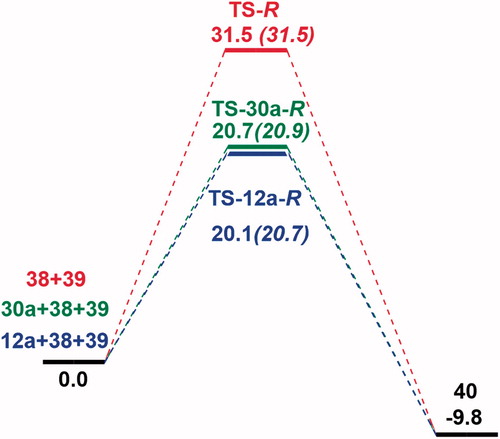
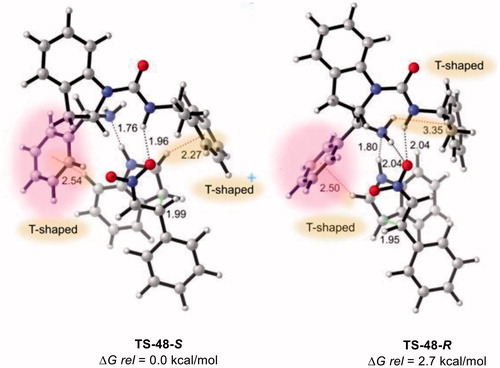
Figure 8. Optimized transition states and activation barriers (ΔG‡) for asymmetric Michael addition of 38 to 39 using catalyst 12a. M06-2X/6-31 + G(d,p) with IEF-PCM in toluene at 298 K and 1 atm, critical distances in Å.
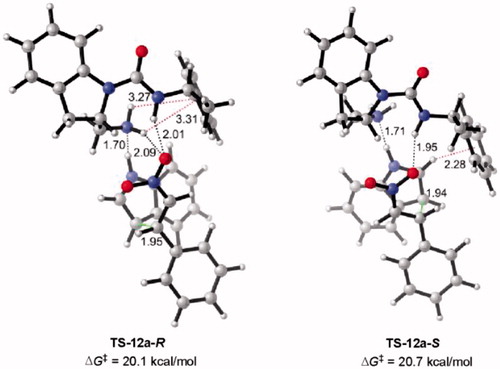
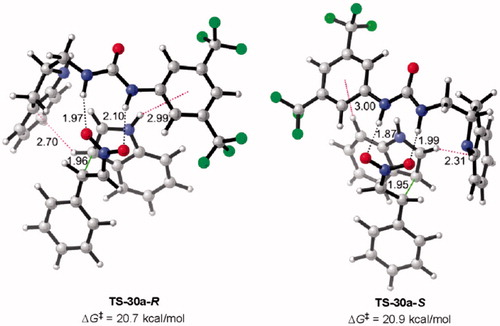
Figure 9. Optimized transition states and activation barriers (ΔG‡) for the Michael addition of 38 to 39 catalyzed by 30a. M06-2X/6-31 + G(d,p) with IEF-PCM in toluene at 298 K and 1 atm, critical distances in Å.