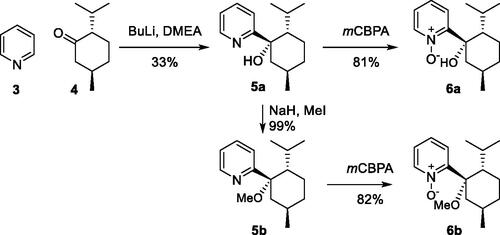
Figures & data
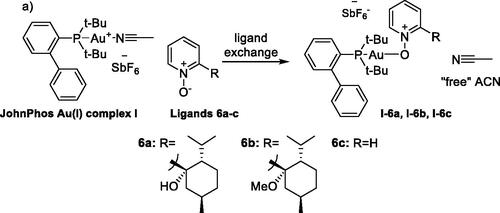
Scheme 3. In situ generation of Au(I)–nitrone complexes (I–6a,b,c) by ligand exchange of JohnPhosAu(I)(ACN)SbF6 complex I with nitrones 6a–c.
Scheme 4. Coordination of nitrone 6a with JohnPhosAu(ACN)SbF6 I, shown as an equilibrium between [complex I + 6a] and [complex I–6a + “free” ACN].
![Scheme 4. Coordination of nitrone 6a with JohnPhosAu(ACN)SbF6 I, shown as an equilibrium between [complex I + 6a] and [complex I–6a + “free” ACN].](/cms/asset/da8e28b0-a497-45dc-8bca-5e05e035af77/lsyc_a_1731550_sch0004_b.jpg)
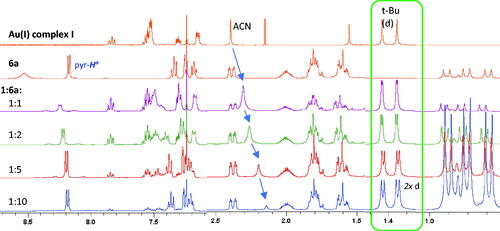
Figure 1. 1H NMR study of nitrones 6a and 6b coordination with JohnPhosAu(ACN)SbF6 I (1:1 in CDCl3).
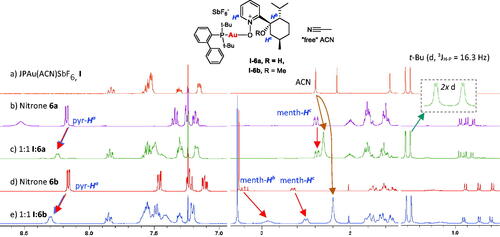
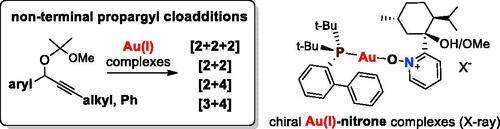
Figure 2. Crystal structure (X-ray) of Au(I)–nitrone complex I–6a. Selected bond lengths (Å) and angles (°) in gold(I)-nitrone complexes I–6a, I–6c and I–6d are given.
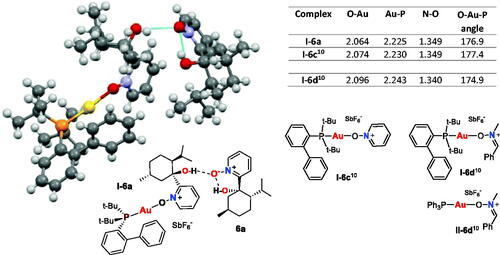
Table 1. Changes in 1H NMR peak shifts, Δδ1HcoordTable Footnotea (ppm), of mixtures with decreasing I:6a ratio.
Table 2. Gold(I)-catalyzed [2 + 2 + 2] cyclotrimerisation of propargyl acetal 1 to trimer 2.
Table 3. Gold(I)-catalyzed dimerization of propargl acetal 10.
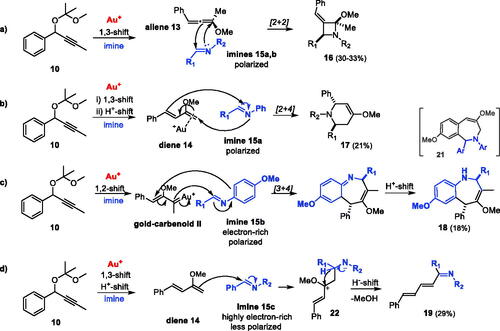
Table 4. Cycloaddition reactions of (a) diene 14 with phenylbenzaldimine[Citation18] and (b) aryl-alkyl-propargyl acetals 10 and 10′ with imines 15a–d.
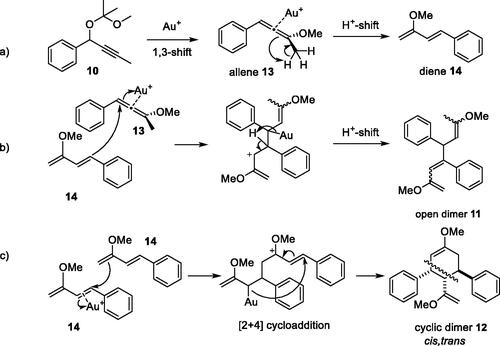
Scheme 5. Proposed pathways for (a) gold-catalyzed generation of allene 13 and diene 14 intermediates from propargyl acetal 10; (b) dimerization of diene 14 with allene 13 to give open dimer 11 and (c) dimerization of two units of diene 14 to give cyclic dimer 12.

![Scheme 1. Gold(I)-nitrone-catalyzed [2 + 2+2] cyclotrimerization of diarylpropargyl acetal 1.](/cms/asset/643e3883-355b-4808-8dea-f880b63e11b3/lsyc_a_1731550_sch0001_b.jpg)