Figures & data
Figure 1. Photomicrographs of neuronal lesions from affected takahē (Porphyrio hochstetteri). (a) A motor neuron of the spinal cord ventral horn has numerous globoid eosinophilic inclusions (H&E; bar = 6 µm). (b) The neuronal globoid inclusions stain with Luxol fast blue (bar = 12 µm). (c) A motor neuron in the brain stem shows a large granular light brown pigment formation (H&E; bar = 12 µm). (d) A ventral horn neuron with granular material at the pole that stains with Luxol fast blue (bar = 10 µm). (e) A ventral horn neuron contains PAS-positive granular material; in contrast the round unstained vacuolar areas are interpreted as globoid bodies as are the empty vacuoles in the neighbouring neuron which do not stain with PAS (bar = 12 µm). (f) A dead ventral horn neuron with eosinophilic globoid bodies and their ghosts (H&E; bar = 5 µm); compare to (a) from the same section. (g) A fluoresent immunostain for lysosomal associated protein 1 (LAMP1) did not stain the large globoid bodies but stained a myriad small bodies in the cytosol indicating their lysosomal nature (bar = 3 µm). (h) The fluorescent immunostain for cathepsin D did not stain the large globoid bodies (arrow) but stained many small bodies in the cytosol (bar = 3 µm). (i) The colourimetric stain (diaminobenzidine; DAB) for LAMP1 stained many small bodies in this ventral horn neuron. Note that in the area of the arrow it stained the periphery of the bodies as expected with LAMP1 primary antibody. The large globoid inclusion did not immunostain (bar = 4 µm). (j) The colourimetric immunostain (DAB) for cathepsin D stained a large number of concentrated small lysosomal bodies at the pole of this ventral horn neuron, which are interpreted as the small granular bodies in other neurons (bar = 4 µm).
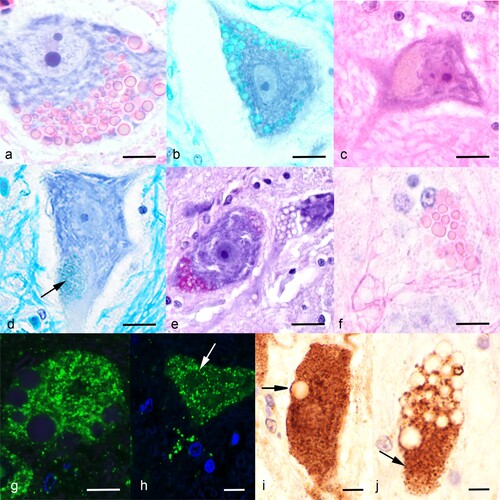
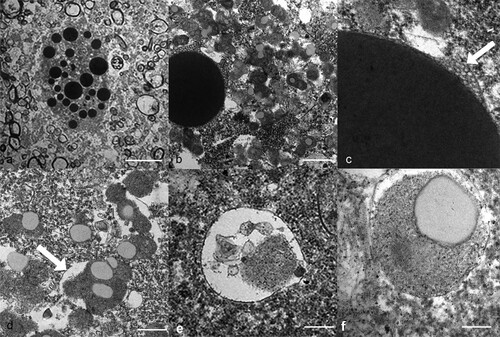
Figure 2. Transmission electron micrographs of the neuronal lesions in a takahē (Porphyrio hochstetteri) (Case 1) with neurological signs. (a) Low power micrograph of large dense globoid bodies of various sizes in the cytosol of a ventral horn neuron (bar = 10 µm). (b) A large electron dense, membrane-bound globoid body is contrasted with numerous small irregular granular bodies in the cytosol (bar = 1 µm). The large body is surrounded by small intraluminal vesicles. (c) High power electron micrograph shows intraluminal vesicles beneath the surrounding membrane (arrow). (d) The electron dense granular bodies are surrounded by a membrane (arrow) and contain electron lucent areas (bar = 500 nm). (e) A vesicle contains some electron dense granular material (bar = 200 nm). (f) A small round granular body within a limiting membrane contains an electron lucent area (bar = 200 nm).