Figures & data
Figure 2. Representative radiochromatograms from 120 min incubations of 3 µg/mL of [14C]-viloxazine with rat, dog, and human hepatocyte incubations.
![Figure 2. Representative radiochromatograms from 120 min incubations of 3 µg/mL of [14C]-viloxazine with rat, dog, and human hepatocyte incubations.](/cms/asset/08cd5666-2678-4ed1-ad14-a64d42a83f76/ixen_a_1767319_f0002_b.jpg)
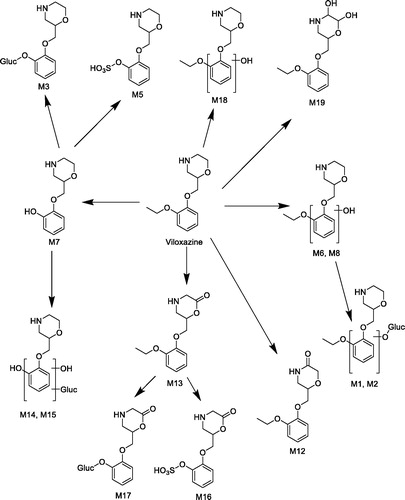
Table 1. Metabolites of [14C]-viloxazine formed in rat, dog, and human hepatocytes following a 120 min incubation. Data expressed as the average percentage of total sample radioactivity.
Figure 3. Metabolite profiles in the plasma (8 h), urine (8 to 24 h), and feces (8 to 24 h) following administration of a single 100-mg/kg oral dose of [14C]-viloxazine to male rats.
![Figure 3. Metabolite profiles in the plasma (8 h), urine (8 to 24 h), and feces (8 to 24 h) following administration of a single 100-mg/kg oral dose of [14C]-viloxazine to male rats.](/cms/asset/316e1f7b-7eb3-4dca-bb75-f024595cc5bd/ixen_a_1767319_f0003_b.jpg)
Table 2. Metabolites of [14C]-viloxazine formed in male (A) and female rats (B) following an oral dose of 100 mg/kg.
Figure 4. Representive chromatogram from human plasma and urine from a single subject following a single oral administration of [14C]-viloxazine (nominal 100 mg; 8.7 MBq) to seven male human subjects. Metabolites P1 and U1 are considered to be the same metabolite and are indicated as such in the plasma chromatogram.
![Figure 4. Representive chromatogram from human plasma and urine from a single subject following a single oral administration of [14C]-viloxazine (nominal 100 mg; 8.7 MBq) to seven male human subjects. Metabolites P1 and U1 are considered to be the same metabolite and are indicated as such in the plasma chromatogram.](/cms/asset/95a86f80-a5c4-48a8-b69a-9e0ed19c7809/ixen_a_1767319_f0004_c.jpg)
Figure 5. Accurate mass extracted ion chromatogram, positive ion full scan, and product ion spectra with a summary of theoretical accurate mass values, characteristic fragment ions, and proposed assignments for the metabolite P1 / U1.
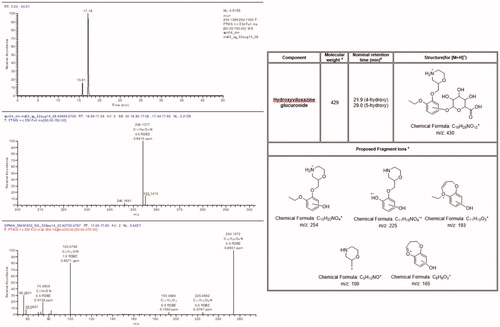
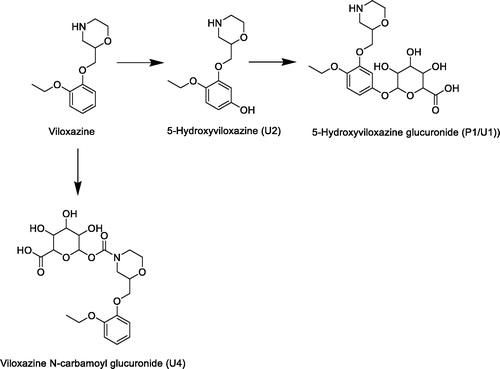
Figure 6. Radiochromatograms and extracted ion chromatograms for selected components from human urine before and after deconjugation with β-glucuronidase to show position of glucuronidation.
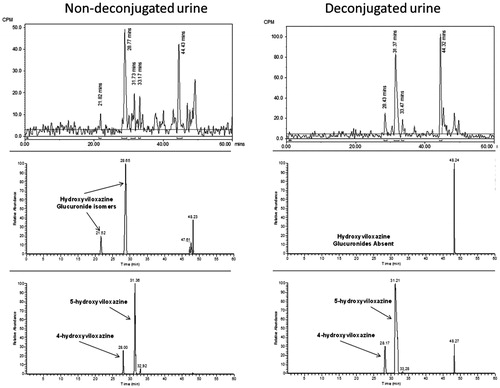
Table 3. Summary of the relative proportions of the human plasma and urinary metabolites following a single oral administration of [14C]-viloxazine (nominal 100 mg; 8.7 MBq) to seven male human subjects.
Figure 7. Formation of 5-hydroxyviloxazine from the incubations of [14C]-viloxazine (40 µM) with a panel of recombinant cytochrome P450s.
![Figure 7. Formation of 5-hydroxyviloxazine from the incubations of [14C]-viloxazine (40 µM) with a panel of recombinant cytochrome P450s.](/cms/asset/eb49e7ae-0204-4fa0-a10a-a920c052cd18/ixen_a_1767319_f0007_b.jpg)
Table 4. Viloxazine in vitro reversible and time dependent CYP inhibition.