Figures & data
Figure 2. Mean concentration-time profiles of radioactivity in plasma and whole blood and lusutrombopag and M5 in plasma after a single oral administration of 2 mg of [14C]-lusutrombopag to healthy subjects. Each point represents the mean from seven subjects. All M3 concentrations were below the limit of quantitation.
![Figure 2. Mean concentration-time profiles of radioactivity in plasma and whole blood and lusutrombopag and M5 in plasma after a single oral administration of 2 mg of [14C]-lusutrombopag to healthy subjects. Each point represents the mean from seven subjects. All M3 concentrations were below the limit of quantitation.](/cms/asset/a260e2e8-2886-4b81-8c87-5405b36b9b1f/ixen_a_1845416_f0002_b.jpg)
Table 1. Pharmacokinetic parameters of lusutrombopag and M5 and total radioactivity in plasma and whole blood after a single oral administration of 2 mg of [14C]-lusutromobpag.
Figure 3. Representative HPLC-radiochromatograms of plasma collected from 8 h, urine collected from 0 to 216 h, and feces collected from 0 to 336 h after single oral administration of 2 mg of [14C]-lusutrombopag to healthy subjects.
![Figure 3. Representative HPLC-radiochromatograms of plasma collected from 8 h, urine collected from 0 to 216 h, and feces collected from 0 to 336 h after single oral administration of 2 mg of [14C]-lusutrombopag to healthy subjects.](/cms/asset/2998857c-2f23-4466-adb9-c6646d0b67c9/ixen_a_1845416_f0003_c.jpg)
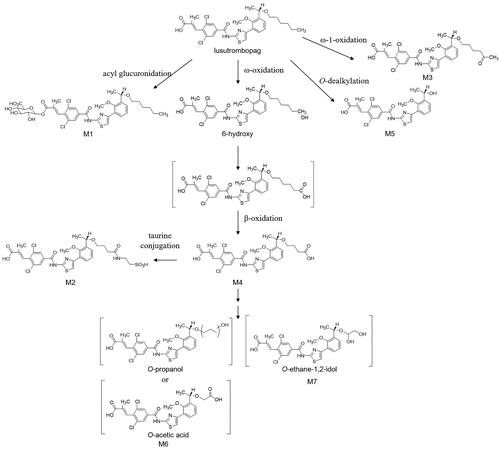
Table 2. Composition of lusutrombopag and its metabolites in urine and feces after a single oral administration of 2 mg of [14C]-lusutromobopag, and assignment of MS/MS fragmentations of unidentified metabolites.
Table 3. In vitro metabolism of [14C]-lusutrombopag by recombinant human cDNA expressed CYP enzymes.
Table 4. Effect of P450 chemical inhibitors on the formation of lusutrombopag-6-hydroxy from [14C]-lusutrombopag following incubation with human liver microsomes.
Table 5. Effect of P450 chemical inhibitors on the formation of lusutrombopag-6-hydroxy and lusutrombopa-β-oxidated carboxylic acid from [14C]-lusutrombopag following incubation with cryopreserved human hepatocytes.

![Figure 1. Chemical structure of [14C]-lusutrombopag, with the site of the 14 C label indicated (*).](/cms/asset/2bafa0eb-65b8-4a38-bc3e-0b7767f3d634/ixen_a_1845416_f0001_b.jpg)