Figures & data
Figure 1. Potential impact of pharmacoenhancers on the time-concentration profile of the co-administered drug. In all cases exposure of the co-administered drug, as measured by AUC, is increased by the pharmacoenhancer. (A) Cmax increases whereas half-life (T½) is unchanged, indicating a reduction in the first pass effect only, which could result from inhibition of gut metabolism (e.g. CYP3A) and/or inhibition of efflux transporters (e.g. P-glycoprotein). (B) Cmax remains the same whilst T½ is increased indicating an increase in systemic clearance due to inhibition of hepatic or extrahepatic metabolism (e.g. CYPs). The impact of a pharmacoenhancer will depend on the route(s) of clearance of the co-administered drug, the potency of the inhibitor and the free concentration of the inhibitor relative to the active drug achieved in the gut and/or liver.
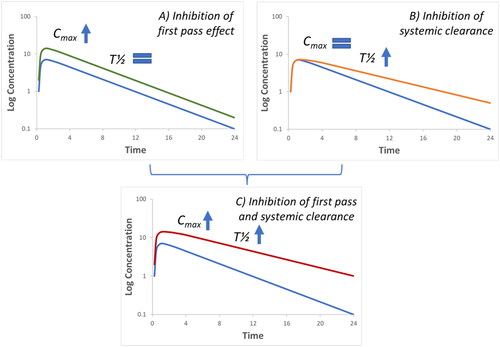
Table 1. Pharmacoenhancer inhibitory potency against principal drug clearance mechanism.
Figure 2. The structure, and Mpro, CYP3A and CYP2D6 inhibitory activity of cobicistat and ritonavir (data from Xu et al. Citation2010).
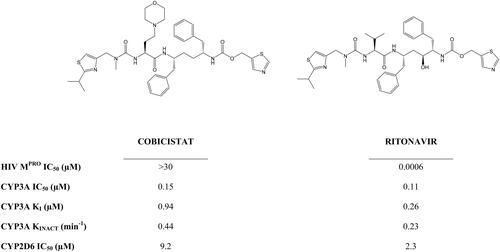
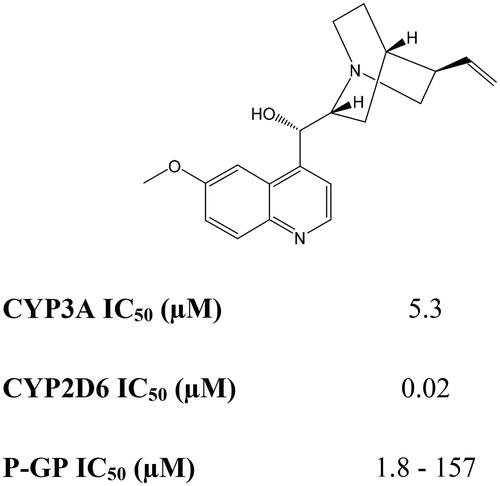
Figure 3. The structure and inhibitory activity of quinidine on CYP2D6, CYP3A4 and P-glycoprotein (data from Galetin et al. Citation2002; Hutzler et al. Citation2003; Morrissey et al. Citation2012).