Figures & data
Figure 1. Metabolic stability of seven test compounds over 48 h in cPHHs. Metabolic stability in cPHHs of (A). ATX (5 µM), (B) GUA (5 µM), (C) L-AMPH (2.5 µM) after racemic AMPH administration, (D) dAMPH (2.5 µM) after racemic AMPH administration, (E) dAMPH after dAMPH administration (5 µM), (F) CLD (5 µM), (G) dMPH (2.5 µM) after racemic MPH administration, (H) dMPH (5 µM) after dMPH administration, and (I) L-MPH (2.5 µM) after racemic MPH administration. AMPH: D/L-amphetamine; ATX: atomoxetine; CLD: clonidine; cPHH: cryopreserved primary human hepatocytes; dAMPH: dextroamphetamine; dMPH: dexmethylphenidate; GUA: guanfacine; L-AMPH: L-amphetamine; L-MPH: L-methylphenidate; MPH: D/L-methylphenidate.
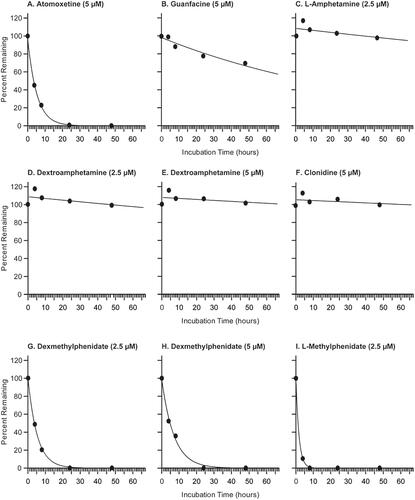
Figure 2. Formation of metabolites over 48 h in cPHHs. The formation of metabolites over 48 h in CPHHs is shown where reference standards were available. (A) Metabolite formation after ATX administration. (B) Metabolite formation after GUA administration. (C) Metabolite formation after racemic AMPH administration. (D) Metabolite formation after dAMPH administration. (E) Metabolite formation after racemic MPH administration. (F) Metabolite formation after dMPH administration. G. Metabolite formation after CLD administration. 3-OH-GUA: 3-hydroxy-guanfacine; 4-OH-AMPH: 4-hydroxy-amphetamine; 4-OH-ATX: 4-hydroxy-atomoxetine; 4-OH-ATX-O-gluc: 4-hydroxy-atomoxetine-O-glucuronide; AMPH: D/L-amphetamine; ATX: atomoxetine; CLD: clonidine; cPHH: cryopreserved primary human hepatocytes; dAMPH: dextroamphetamine; dMPH: dexmethylphenidate; MPH: D/L-methylphenidate; GUA: guanfacine; L-AMPH: L-amphetamine; L-MPH: L-methylphenidate; MPH: D/L-methylphenidate; N-desmethyl-ATX: N-desmethyl-atomoxetine.
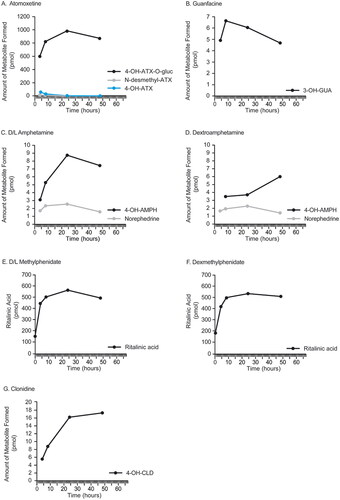
Table 1. Substrate depletion, half-life and intrinsic clearance in cPHHs.
Table 2. Substrate loss in inhibitor-free HLMs.
Table 3. CYP involvement in metabolism identified by specific inhibitors with HLMs.
Table 4. FMO involvement in metabolism identified by heat-inactivated HLMs.
