Figures & data
Figure 1. Structure of vatiquinone and metabolic pathways following oral dose administration in rats, dogs, and human subjects.
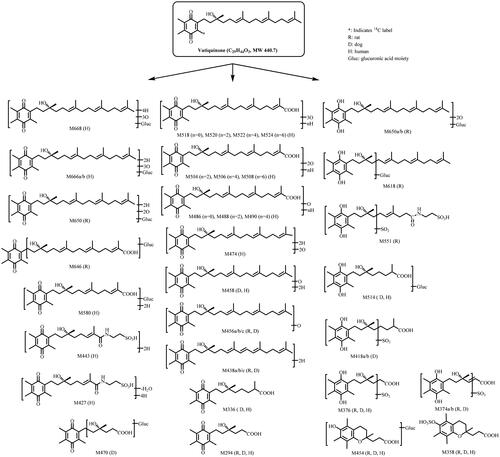
Table 1. Pharmacokinetic parameters of 14C-vatiquinone-derived radioactivity in plasma and tissues following a 30 mg/kg 14C-vatiquinone oral dose in male Long-Evans rats.
Figure 2. Representative HPLC radio-chromatograms of 2 h (A), 4 h (B), 8 h (C), 12 h (D), and 24 h (E) plasma from intact rats following a single 300 mg/kg oral dose of 14C-vatiquinone.
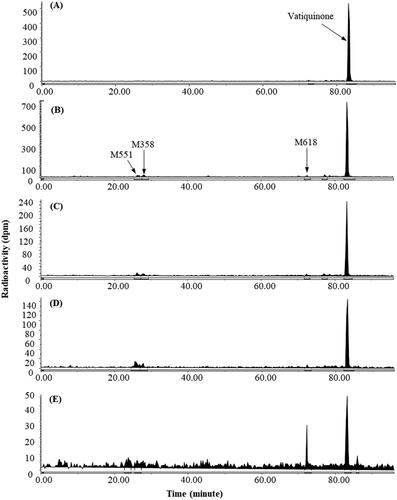
Figure 3. Representative HPLC radio-chromatograms 0–48 h bile (A), 0–72 h urine (B), and 0–48 h faeces (C) from bile-duct cannulated rats, and 0–48 h urine (D) and 0–48 h faeces (E) from intact rats following a single 300 mg/kg oral dose of 14C-vatiquinone.
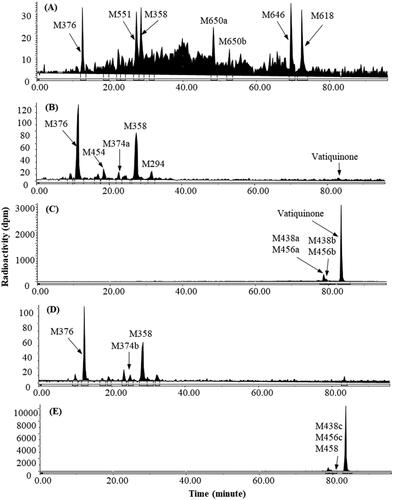
Table 2. Percentage of the administered dose recovered following a single oral dose of 14C-vatiquinone in male Sprague Dawley rats, male Beagle dogs, and male human subjects.
Table 3. Pharmacokinetic parameters for plasma metabolites following a single oral dose of 14C-vatiquinone in male Sprague Dawley rats, male Beagle dogs, and male human subjects.
Table 4. Percentage of dose excreted as vatiquinone and metabolites in pooled bile, urine, and faeces following a single oral dose of 14C-vatiquinone in male Sprague Dawley rats, male Beagle dogs, and male human subjects.
Figure 4. Representative HPLC radio-chromatograms of 1 h (A), 2 h (B), 4 h (C), 6 h (D), 12 h (E), 24 h (F), 48 h (G), and 96 h (H) plasma, 0–144 h urine (I) and 0–48 h faeces (J) from dogs following a single 100 mg/kg oral dose of 14C-vatiquinone.
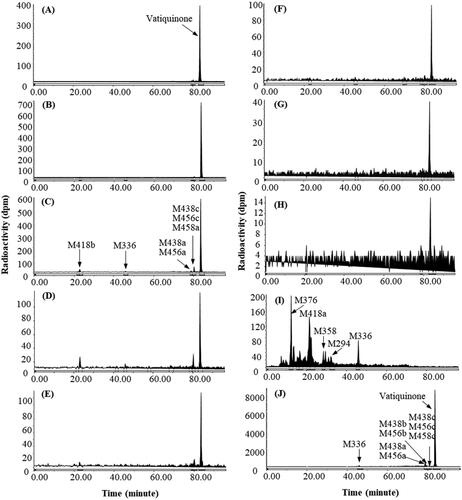
Figure 5. Representative HPLC radio-chromatograms of 2 h (A), 4 h (B), 6 h (C), 8 h (D), 24 h (E), and 48 h (F) plasma, 0–24 h urine (G), and 0–72 h faeces (H) from human subjects following a single 400 mg oral dose of 14C-vatiquinone.
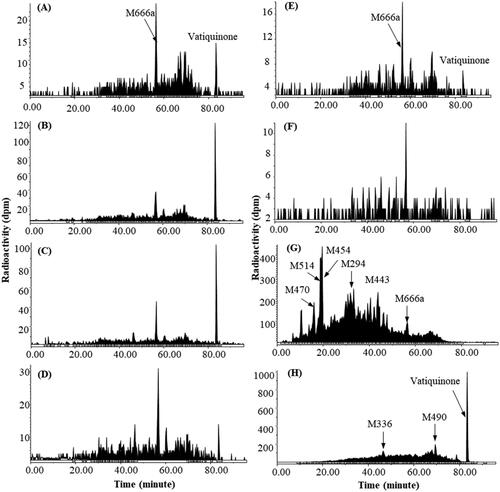
Figure 6. Plasma concentration–time curves of total radioactivity (TRA), vatiquinone, and its metabolites following oral dose administration in rats (A), dogs (B), and human subjects (C).
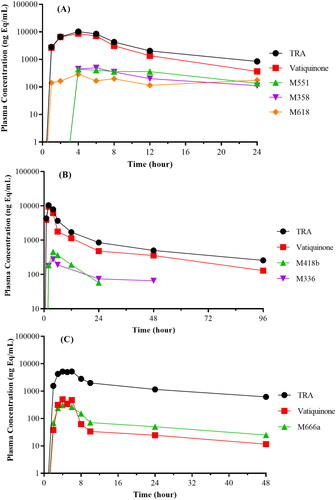
Table 5. Mass spectral analysis of major metabolites of vatiquinone in plasma, bile, urine, and faeces from rats, dogs, and human subjects.
