Figures & data
Figure 1. (A) Structure of 2,2′-bipyridine. (B) Structure of 2-(benzo[d]oxazol-2-yl) nicotinic acid.
![Figure 1. (A) Structure of 2,2′-bipyridine. (B) Structure of 2-(benzo[d]oxazol-2-yl) nicotinic acid.](/cms/asset/cd2c7f47-aa9b-4e3d-8f01-52acb9df398e/gcoo_a_2249582_f0001_b.jpg)
Table 1. Reported stability constants of the hydroxo complexes of Cu(II) at 25 °C and KNO3 1 M as ionic medium [Citation23].
Table 2. Reported stability constants of the binary complexes formed in the systems Cu(II)–HSer, Cu(II)–HMet, Cu(II)–HPhe and Cu(II)–HThr at 25 °C and KNO3 1 M as ionic medium [Citation23].
Table 3. Reported stability constants of the binary complexes formed in the systems Cu(II)–H2Asp, Cu(II)–H2Glu and Cu(II)–HHis at 25 °C and KNO3 1 M as ionic medium [Citation23].
Table 4. Reported stability constants of the binary complexes formed in the system Cu(II)– HOxa at 25 °C and KNO3 1 M as ionic medium [Citation20].
Table 5. Ternary species with corresponding formation constants for the H+-Cu(II)-L-Ser, H+-Cu(II)-L-Thr, H+-Cu(II)-L-Met and H+-Cu(II)-L-Phe systems at KNO3 1.0 M and 25 °C.
Table 6. Ternary species with corresponding formation constants for the H+-Cu(II)-L-Asp, H+-Cu(II)-L-Glu and H+-Cu(II)-L-His systems at KNO3 1.0 M and 25 °C.
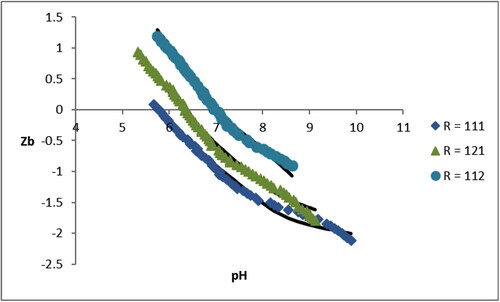
Figure 3. Species distribution diagram for the systems: (A) Cu(II)-L-His, (B) Cu(II)-L-Ser and (C) Cu(II)-L-Glu[Cu(II)] = 3.0 mM, R = 1:1:1.
![Figure 3. Species distribution diagram for the systems: (A) Cu(II)-L-His, (B) Cu(II)-L-Ser and (C) Cu(II)-L-Glu[Cu(II)] = 3.0 mM, R = 1:1:1.](/cms/asset/69ccb21c-7885-4307-a3a3-d206372f3f3d/gcoo_a_2249582_f0003_c.jpg)
Table 7. Δlog K and log χ values calculated for the system studied.
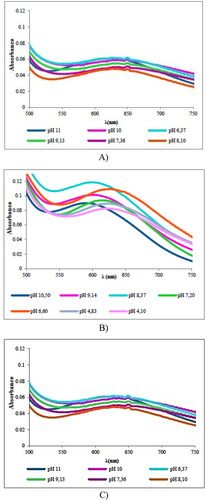
Figure 4. UV–vis spectra at different pH values of the systems: (A) Cu(II)-L-His, (B) Cu(II)-L-Ser and (C) Cu(II)-L-Glu.
gcoo_a_2249582_sm7792.docx
Download MS Word (106.3 KB)Availability of data and materials
Not applicable.