Figures & data
Figure 1. Short-term removal of aqueous Mn(II) by viable S. putrefaciens in absence/presence of equal concentration of fulvic acids (A) and alteration of pH in the same experimental batches (B).
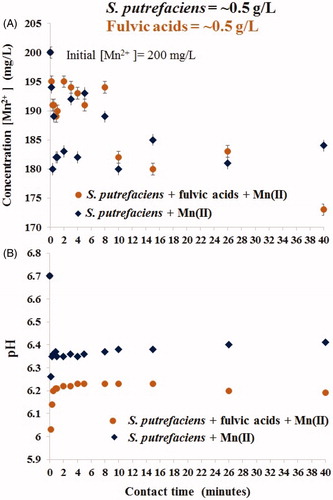
Figure 2. Long-term uptake of aqueous Mn(II) by S. putrefaciens in absence/presence of equal concentration of fulvic acids (A) and drift of pH in the same experiments (B).
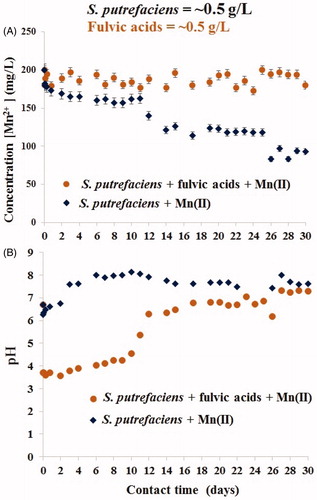
Table 1. Adsorptive (q, mg/gdw) and removal (%) characteristics of viable S. putrefaciens of the density (adsorbent dose) of 0.5 and 1.0 gdw/L at Mn2+ initial concentration of 200 mg/L.
Figure 3. Kinetics of Mn(II) sorption by viable S. putrefaciens (1 gdw/L) for 30 min in absence/presence of fulvic acids (45 mg/L) at the initial Mn(II) concentration of 200 mg/L (A) and drift of pH in the same batches (B).
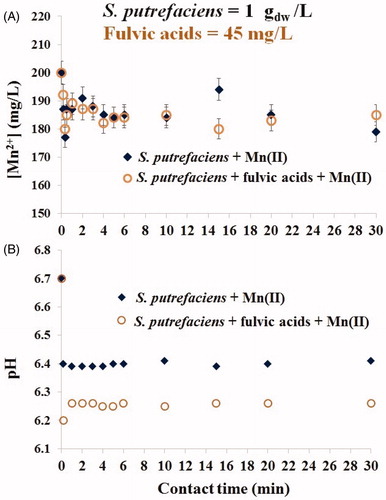
Figure 4. Kinetics of Mn(II) sorption by viable S. putrefaciens (1 gdw/L) for 38 days in absence/presence of fulvic acids (45 mg/L) at the initial Mn(II) concentration of 200 mg/L (A) and change of pH in the same experiments (B).
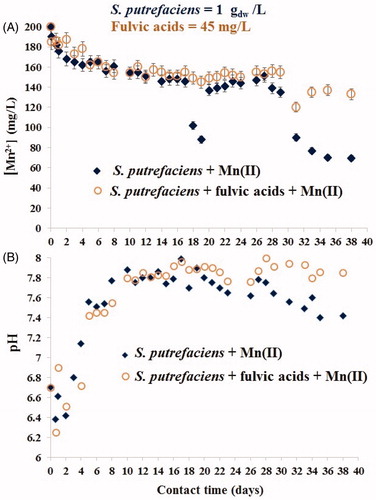
Figure 5. FTIR spectra of initial S. putrefaciens and contacted to aqueous Mn2+ in 0.1 M NaCl electrolyte for three days.
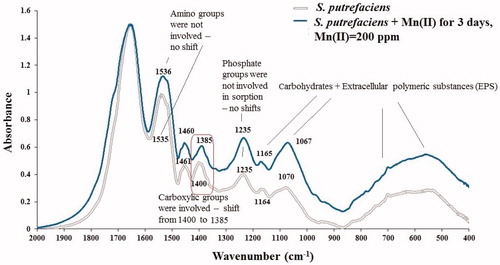
Figure 6. FTIR spectra of initial S. putrefaciens (1 gdw/L), contacted to Mn2+ in absence/presence of fulvic acids (45 mg/L) for 38 days and fulvic acids used in this study (A), and of two references, Mn3(PO4)2 (B) and MnCO3 (C).
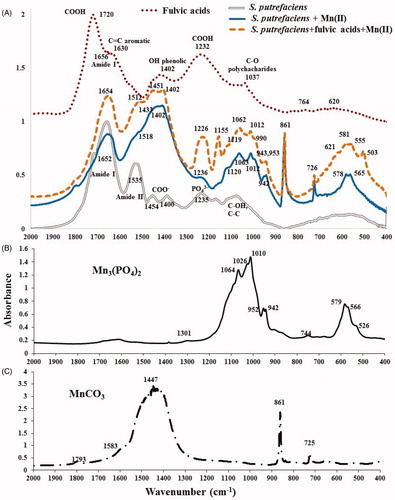
Table 2. Characteristic (400–2000 cm−1) IR-vibrations in experimental samples of original S. putrefaciens (1 gdw/L) and influenced by aqueous Mn2+ in absence/presence of fulvic acids (shown in ).
Table 3. C 1 s and N 1 s XPS features in four samples: uncontacted S. putrefaciens and with Mn(II) sorbed in absence/presence of fulvic acids over 38 days, and fulvic acids.
Table 4. XPS features (O 1 s, Mn 2p, Na 1 s, P 2p, Cl 2p and S 2p) in four samples: initial S. putrefaciens and with Mn(II) sorbed in absence/presence of fulvic acids over 38 days, and fulvic acids.
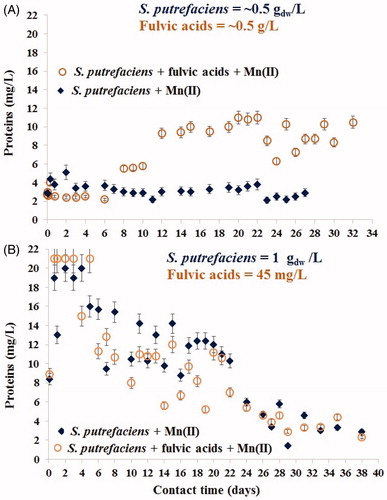
Figure 7. Concentrations of dissolved protein-like substances released by viable S. putrefaciens for one month contact with aqueous Mn2+ in absence/presence of very high (A) and low (environmentally relevant) (B) contents of fulvic acids.