Figures & data
Table 1. Description of the study population.
Figure 1. Percentage of participants with a sufficient, insufficient or deficient vitamin D status. Vitamin D status in breast cancer patients (n = 95) and women without cancer (n = 52) according to the time of sample collection. Baseline samples were collected before start of chemotherapy (CTx) for patients with breast cancer.
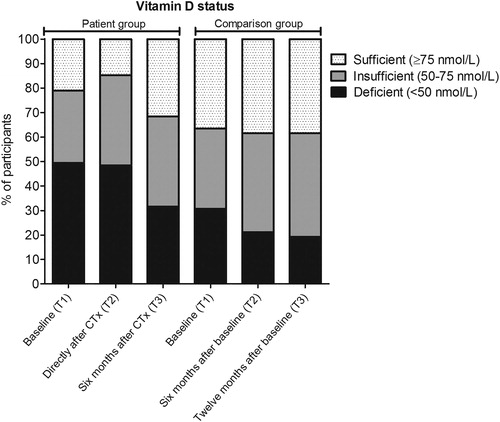
Figure 2. Changes in circulating 25(OH)D3 levels. Estimated marginal means (95% confidence intervals) of the circulating 25(OH)D3 levels throughout the course of chemotherapy (CTx) in patients with breast cancer and at comparable time points for women from the comparison group based on a linear mixed model analysis. The analyses were adjusted for age and season of each blood withdrawal. aStatistically significant interaction for time*group in the linear mixed model analysis. *Indicates a statistically significant difference (Sidak-adjusted P < 0.05) for the comparison between patients and women without cancer identified through a pairwise post-hoc analysis. **Indicates a statistically significant change over time in the patients with breast cancer (Sidak-adjusted P < 0.05). A] All patients with breast cancer (n = 95) and women without cancer (n = 52), B] Patients receiving adjuvant chemotherapy (n = 62) and women without cancer (n = 52), C] Patients receiving neoadjuvant chemotherapy (n = 33) and women without cancer (n = 52), D] Pre-menopausal patients (n = 53) and women without cancer (n = 29), E] Post-menopausal patients (n = 40) and women without cancer (n = 22).
![Figure 2. Changes in circulating 25(OH)D3 levels. Estimated marginal means (95% confidence intervals) of the circulating 25(OH)D3 levels throughout the course of chemotherapy (CTx) in patients with breast cancer and at comparable time points for women from the comparison group based on a linear mixed model analysis. The analyses were adjusted for age and season of each blood withdrawal. aStatistically significant interaction for time*group in the linear mixed model analysis. *Indicates a statistically significant difference (Sidak-adjusted P < 0.05) for the comparison between patients and women without cancer identified through a pairwise post-hoc analysis. **Indicates a statistically significant change over time in the patients with breast cancer (Sidak-adjusted P < 0.05). A] All patients with breast cancer (n = 95) and women without cancer (n = 52), B] Patients receiving adjuvant chemotherapy (n = 62) and women without cancer (n = 52), C] Patients receiving neoadjuvant chemotherapy (n = 33) and women without cancer (n = 52), D] Pre-menopausal patients (n = 53) and women without cancer (n = 29), E] Post-menopausal patients (n = 40) and women without cancer (n = 22).](/cms/asset/3ae1c0fd-4b4c-4833-a470-5ee1a15d7e48/hnuc_a_1559938_f0002_b.jpg)
Table 2. Changes in 25OHD levels over time.
Figure 3. Use of dietary supplements containing vitamin D. Dietary supplements with vitamin D as well as multivitamins containing vitamin D were considered. Use of these supplements was reported by the participants at start of the study (T1) and 6 months after the end of chemotherapy for patients (i.e. 12 months after start of the study for the comparison group) (T3). Consistency is reflecting use or nonuse of these supplements at the indicated time points, whereas inconsistency refers to changes in dietary supplement use (i.e., starting of stopping use of dietary supplements containing vitamin D). A] Patients with breast cancer (n = 95), B] Women without cancer from the comparison group (n = 52).
![Figure 3. Use of dietary supplements containing vitamin D. Dietary supplements with vitamin D as well as multivitamins containing vitamin D were considered. Use of these supplements was reported by the participants at start of the study (T1) and 6 months after the end of chemotherapy for patients (i.e. 12 months after start of the study for the comparison group) (T3). Consistency is reflecting use or nonuse of these supplements at the indicated time points, whereas inconsistency refers to changes in dietary supplement use (i.e., starting of stopping use of dietary supplements containing vitamin D). A] Patients with breast cancer (n = 95), B] Women without cancer from the comparison group (n = 52).](/cms/asset/26dfe728-b251-4bff-a32f-3b155c9e5b84/hnuc_a_1559938_f0003_b.jpg)
