Figures & data
Table 1. Summary of the flock data, post-mortem analysis and bacteriology of the 2004 and 2005E. hirae-associated endocarditis outbreaks.
Figure 1. Daily mortality percentage from day 0 to day 33 for flocks A4, K, I2 and E2, diagnosed with endocarditis in 2005, compared to unaffected flocks of the same farms (note the increased mortality of endocarditis flocks between day 9 and 22).
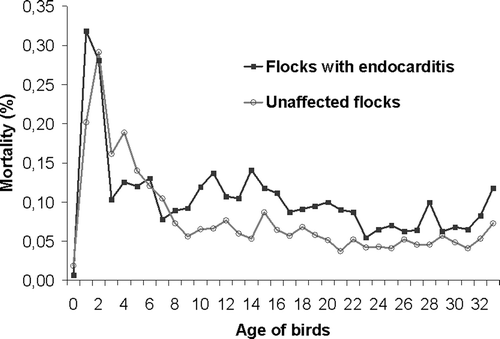
Figure 2. Gross lesions in birds with E. hirae-associated endocarditis with (a) an intact heart with a more pronounced rounded shape, due to vegetative lesions of the right AV valve and (b) inner aspect of the same heart with vegetative lesions of the right AV valve.
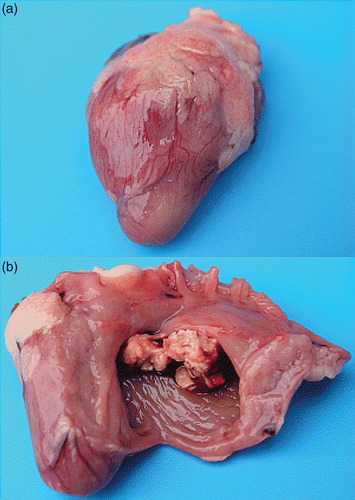
Figure 3. Thrombotic lesions in birds with E. hirae-associated endocarditis with (a) a thrombus in a large branch of the A. pulmonalis of the lung (arrow) and (b) a thrombus in the left A. ischiadica externa (arrow).
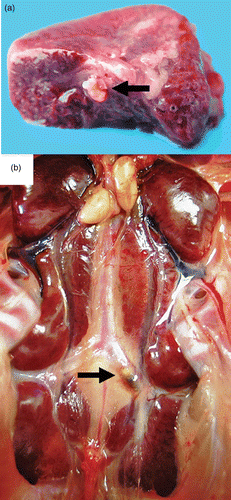
Table 2. Odds Ratios and 95% confidence intervals for the associations between localization of endocarditis lesions in the heart and presence of other lesions in birds of Flocks A4, K, L1/L2, and E2 of the 2005 outbreak.
Table 3. Distribution of lesions in the hearts of succumbed broilers of flocks A4, K, L1/L2, I2 and E2 per week of the grow-out period from day 15 onwards.
Figure 4. Haematoxylin and eosin sections of hearts with E. hirae-associated endocarditis showing (a) fibrinous thromboendocarditis of the right atrioventricular valve: the vegetative lesion is covered by a thrombus with erythrocytes, fibrin and accumulations of cocci, mural attachment of the vegetative process is present and the myocardium seems unaffected (bar = 100 µm), and (b) a subendocardial lesion, with numerous cocci, embedded in fibrillar eosinophilic proteinaceous material (fibrin): some inflammatory cells are present and the myocardium seems unaffected (bar = 20 µm).
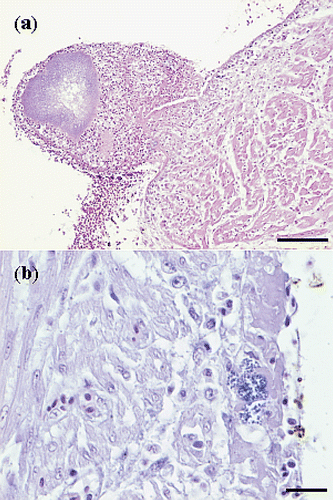
Figure 5. Haematoxylin and eosin sections of thrombotic lesions in the A. pulmonalis of the lungs from birds with E. hirae-associated endocarditis, with (a) thrombosis and endarteritis of a large branch of the A. pulmonalis, with mural attachment, containing accumulations of small cocci (bar = 500 µm), and (b) thrombosis and endarteritis in a large branch of the A. pulmonalis: the smaller branches of the A. pulmonalis following the subdivisions of the bronchi and the capillary network in the parabronchi are unaffected (bar = 500 µm).
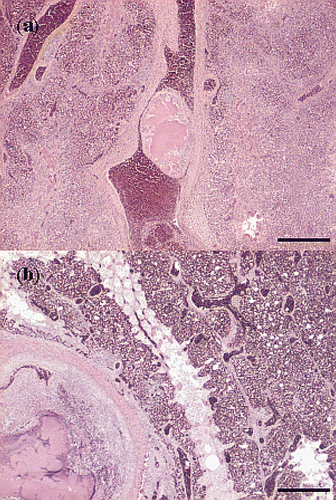
Figure 6. Haematoxylin and eosin sections of thrombotic lesions in the liver from birds with E. hirae-associated endocarditis. with (a) thrombosis of a periportal vein: insert showing a thrombus being composed of fibrin and debris and disintegrating heterophils present in the thrombus (bar = 100 µm), and (b) subacute process consisting of focal coagulative necrosis with heterophil infiltration and large mesenchymal cells, possibly activated Kupffer cells, and infarction with erythrocytes: insert showing necrosis, mesenchymal cells and heterophils (bar = 200 µm).
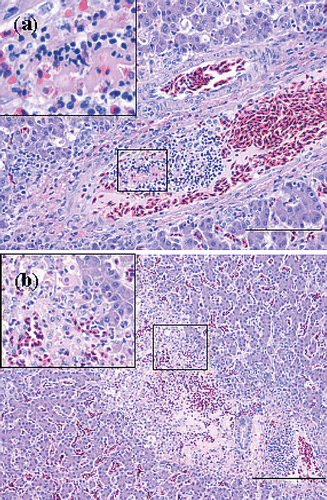
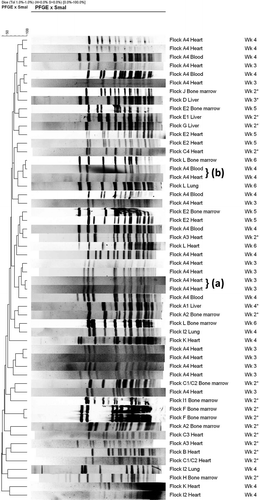
Figure 7. PFGE banding patterns for isolates of E. hirae from heart, liver, bone marrow, blood and lungs from affected flocks from the 2004 (denoted with *) and 2005 outbreak at different times during the grow-out period. Genetically closely related isolates are present within some flocks, but not between flocks, and mostly originated from the same source, i.e. heart (a). Some genetic relatedness was present in isolates from the same flock but of different sources, i.e. heart and blood (b).