Figures & data
Figure 1. (A) Hippo On. Upon a plethora of external signals, such as G-protein coupled receptors, cell-adhesion, and (mechano)stress, MST1/2 (mammalian homologue of Drosphilia Hippo), and SAV1 are phosphorylated upon which LATS1/2-MOB protein become phosphorylated. This leads to phosphorylation of YAP/TAZ, that upon binding to 14-3-3 proteins degrades. (B) Hippo OFF. Due to lack of stimuli on MST1/2-SAV1, these protein remain unphosphorylated, as well as downstream proteins LATS1/2-MOB and YAP/TAZ. Unphosphorylated YAP/TAZ can enter the nucleus where, upon interaction ioth TEAD dries the transcription of specific gene products, such as cyr61, ctgf and nuak2.
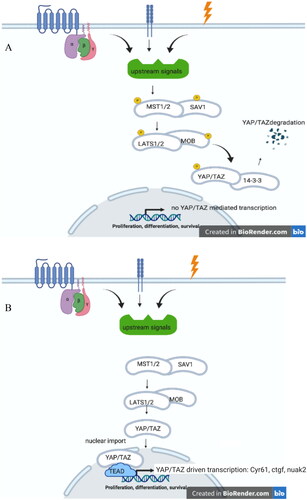
Table 1. Drug affecting YAP/TAZ-activation.
