Figures & data
Figure 1. Innate immune response against H9N2 AIV infection in chicken. H9N2 virus is recognized by melanoma differentiation-associated gene 5 (MDA5) and asp–-glu–-ala–-asp (DEAD)-box polypeptide 3 X-linked (DDX3X) which trigger downstream pathways through the stimulator of IFN genes (i.e. STING) and the mitochondrial antiviral-signaling protein (MAVS), respectively. Although MAVS also activates STING after receiving the upstream signal, what remains uncertain is whether MDA5 directly activates STING. In any case, STING activates the transcription factors IFN regulatory factor 7 (IRF7) and nuclear factor kappa B (NF-κB) by orchestrating the assembly of TANK-binding kinase 1 (TBK1). even so, the interaction between TBK1 and IRF7 also remains unclear. Once activated, IRF7 and NF-κB translocate into the nucleus and are phosphorylated, which further stimulates the transcription of type 1 interferons and proinflammatory factors. In turn, type I IFNs stimulate the production of the IFN-stimulated gene factor (ISG) by both autocrine and paracrine signaling through cognate type I IFN receptor recognition.
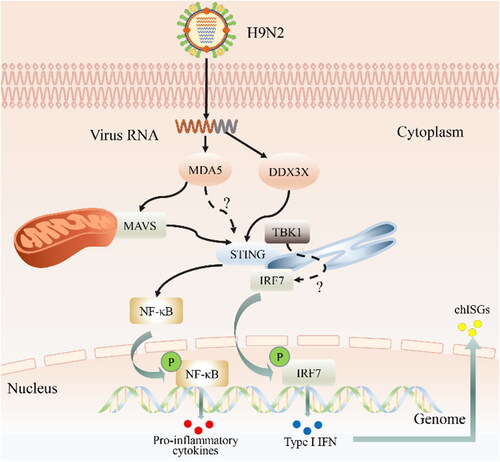
Figure 2. The Possible adaptive immune response against H9N2 AIV infection in chicken. The mechanisms of cytokines and signaling molecules in adaptive immunity remain largely elusive in chickens, so relevant studies of mice and humans are cited to furnish explanations. After the H9N2 virus infects chickens, it is recognized by antigen-presenting cells (APCs), among which DCs are the most important. DCs present extracellular and intracellular antigens to naive CD4 and CD8 T cells after capturing and processing antigens, respectively. CD4 T cells subsequently differentiate into Th1, Th2, and Th17 cells. Th2 cells are induced by autocrine IL-4 from naive CD4 T cells. Th1 cells are induced by IL-12 and IL-18 secreted by DCs. Th1 cells promote the activation of cytotoxic T lymphocyte (CTL) cells, which induce cytotoxicity by secreting IL-2 and IFN-γ, and, then granzyme and perforin are important effector molecules of CTL cells. Meanwhile, Th2 cells secrete IL-4 and IL-5, which promote the production of virus-specific antibodies by B cells.