Figures & data
Figure 1. Genetic elimination of BK channels increases synaptic transmission tolerance to acute oxidative stress. (A) Average time to synaptic failure is prolonged in Slo4 mutants compared to w1118 control larvae during H2O2 exposure [one-way ANOVA, F(3, 16) = 17.76, p < .05]. Vertical bar chart is shown as mean ± SEM. Different letters on bar charts indicate statistical significance, whereas the same letter indicates a nonsignificant difference. (B) Average amplitude decline of evoked EJPs in w1118 controls and Slo4 mutants in the presence and absence of 2.25 mM H2O2 (n = 4–6 preparations per group). Black arrows indicate time to synaptic failure of individual w1118 control larval preparations in HL3 saline. Each data point is displayed as mean ± SEM. (C) Representative waveforms of evoked EJPs from w1118 control larvae 1 min before H2O2 exposure (black line) and 10 min after H2O2 exposure (gray line). (D) Representative waveforms of evoked EJPs from Slo4 mutant larvae 1 min before H2O2 exposure (black line) and 10 min after H2O2 exposure (gray line). H2O2 does not acutely affect characteristic EJP shape in both w1118 control and Slo4 mutant larvae.
![Figure 1. Genetic elimination of BK channels increases synaptic transmission tolerance to acute oxidative stress. (A) Average time to synaptic failure is prolonged in Slo4 mutants compared to w1118 control larvae during H2O2 exposure [one-way ANOVA, F(3, 16) = 17.76, p < .05]. Vertical bar chart is shown as mean ± SEM. Different letters on bar charts indicate statistical significance, whereas the same letter indicates a nonsignificant difference. (B) Average amplitude decline of evoked EJPs in w1118 controls and Slo4 mutants in the presence and absence of 2.25 mM H2O2 (n = 4–6 preparations per group). Black arrows indicate time to synaptic failure of individual w1118 control larval preparations in HL3 saline. Each data point is displayed as mean ± SEM. (C) Representative waveforms of evoked EJPs from w1118 control larvae 1 min before H2O2 exposure (black line) and 10 min after H2O2 exposure (gray line). (D) Representative waveforms of evoked EJPs from Slo4 mutant larvae 1 min before H2O2 exposure (black line) and 10 min after H2O2 exposure (gray line). H2O2 does not acutely affect characteristic EJP shape in both w1118 control and Slo4 mutant larvae.](/cms/asset/146c5645-c6ca-4b6f-aec4-d4848776db9a/ineg_a_1500571_f0001_b.jpg)
Figure 2. Drosophila muscle resting membrane potential (RMP) and EJP amplitude decline during acute oxidative stress. (A) RMP from Drosophila larval muscle 6 was recorded in w1118 control larvae and Slo4 mutants in the presence and absence of 2.25 mM H2O2 (n = 6 per group). RMP depolarization occurs more rapidly in w1118 and Slo4 mutant larvae exposed to 2.25 mM H2O2 compared to HL3 control larvae. (B) In the same larval preparations, EJP amplitude was also recorded (n = 6 per group). Loss of RMP tracks EJP amplitude decline across all treatments.
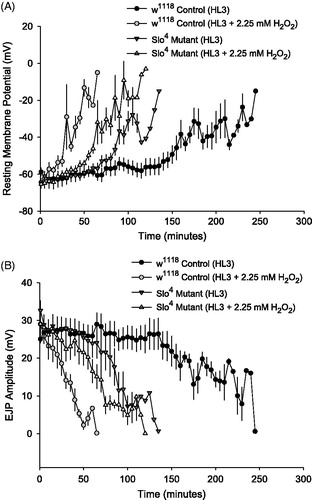
Figure 3. PKG functions independently of BK channels to protect synaptic function from H2O2 exposure. PKG activation using 40 µM 8-bromo-cGMP [one-way ANOVA, F(9, 53) = 13.24, p < .001] and 40 µM sildenafil citrate [p < .001] in Slo4 mutant larvae significantly decreases time to synaptic failure during acute oxidative stress. Activation of PKG with 40 µM 8-bromo-cGMP [p = .651] and 40 µM sildenafil citrate [p = .313] does not alter time to synaptic failure in w1118 control larvae. Activation of voltage-gated K+ channels using 0.25 mM DCA in w1118 control larvae does not alter time to synaptic failure during acute oxidative stress [p = .561]. Application of 0.25 mM DCA to Slo4 mutant larval preparations decreases time to synaptic failure during oxidative stress [p < .05]. Vertical bar chart is shown as mean ± SEM. n = 5–9 preparations per group.
![Figure 3. PKG functions independently of BK channels to protect synaptic function from H2O2 exposure. PKG activation using 40 µM 8-bromo-cGMP [one-way ANOVA, F(9, 53) = 13.24, p < .001] and 40 µM sildenafil citrate [p < .001] in Slo4 mutant larvae significantly decreases time to synaptic failure during acute oxidative stress. Activation of PKG with 40 µM 8-bromo-cGMP [p = .651] and 40 µM sildenafil citrate [p = .313] does not alter time to synaptic failure in w1118 control larvae. Activation of voltage-gated K+ channels using 0.25 mM DCA in w1118 control larvae does not alter time to synaptic failure during acute oxidative stress [p = .561]. Application of 0.25 mM DCA to Slo4 mutant larval preparations decreases time to synaptic failure during oxidative stress [p < .05]. Vertical bar chart is shown as mean ± SEM. n = 5–9 preparations per group.](/cms/asset/21c6ad0b-9611-4e2c-8e7f-6496a5541f5e/ineg_a_1500571_f0003_b.jpg)
Figure 4. Pharmacological blockade of BK channel conductance protects synaptic transmission from acute stress. Pharmacological inhibition of BK channel conductance using 500 pM iberiotoxin significantly increases time to synaptic failure in w1118 control larvae exposed to 2.25 mM H2O2 compared to w1118 H2O2 controls [one-way ANOVA, F(3, 19) = 9.449, p < .05]. Nonselective K+ channel inhibition using 0.25 mM TEA also increases synaptic transmission tolerance to acute oxidative stress in w1118 control larvae [p < .05]. Vertical bar chart is shown as mean ± SEM. n = 5–6 preparations per group.
![Figure 4. Pharmacological blockade of BK channel conductance protects synaptic transmission from acute stress. Pharmacological inhibition of BK channel conductance using 500 pM iberiotoxin significantly increases time to synaptic failure in w1118 control larvae exposed to 2.25 mM H2O2 compared to w1118 H2O2 controls [one-way ANOVA, F(3, 19) = 9.449, p < .05]. Nonselective K+ channel inhibition using 0.25 mM TEA also increases synaptic transmission tolerance to acute oxidative stress in w1118 control larvae [p < .05]. Vertical bar chart is shown as mean ± SEM. n = 5–6 preparations per group.](/cms/asset/61c8b311-5307-4cb6-9700-45022edf3083/ineg_a_1500571_f0004_b.jpg)
Figure 5. Iberiotoxin does not possess major off-target effects using this experimental model. Administration of BK channel inhibitor iberiotoxin does not significantly alter time to synaptic failure in Slo4 mutant larvae [one-way ANOVA, F(2, 16), p = .736], suggesting that iberiotoxin does not possess any major off-target effects at the Drosophila larval NMJ. Vertical bar chart is shown as mean ± SEM. n = 4–6 preparations per group.
![Figure 5. Iberiotoxin does not possess major off-target effects using this experimental model. Administration of BK channel inhibitor iberiotoxin does not significantly alter time to synaptic failure in Slo4 mutant larvae [one-way ANOVA, F(2, 16), p = .736], suggesting that iberiotoxin does not possess any major off-target effects at the Drosophila larval NMJ. Vertical bar chart is shown as mean ± SEM. n = 4–6 preparations per group.](/cms/asset/bc090c7b-2187-42be-ab85-0672c815a93d/ineg_a_1500571_f0005_b.jpg)
