Figures & data
Figure 1. PI4KII restrains nerve terminal growth. (A, C) Representative images of fixed third instar larval nmjs stained with an FITC-conjugated anti-HRP antibody and anti-brp antibody. (B) There was a significant increase (p < .001) in the number of 1b and 1s boutons in PI4KII null mutants (n = 14) in comparison to controls (w1118; n = 15). (D) There was a significant increase (p < .01) in the number of 1b and 1s boutons in nmjs of larvae expressing PI4KIIATP in a PI4KII null mutant background (n = 7) in comparison to larvae expressing PI4KIIwt in a PI4KII null mutant background (n = 9). Error bars represent SEM.
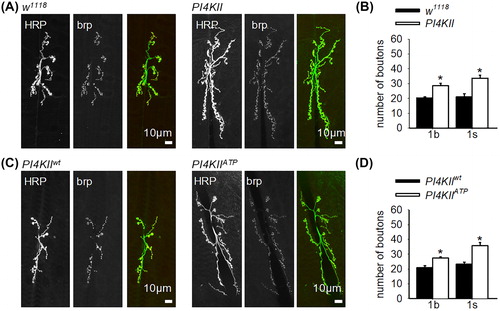
Figure 2. Evoked and spontaneous neurotransmitter release are not affected by the absence of PI4KII. (A) Representative traces of EJPs and mEJPs in controls (w1118) and PI4KII null mutants. (B) There was no significant difference (p > .05) in EJP amplitude in response to 0.05 Hz stimulation between controls (w1118; n = 7) and PI4KII null mutants (n = 5). (C, D) There were no significant differences in the amplitude or frequency of mEJPs between controls (w1118; n = 7) and PI4KII null mutants (n = 5). Error bars represent SEM.
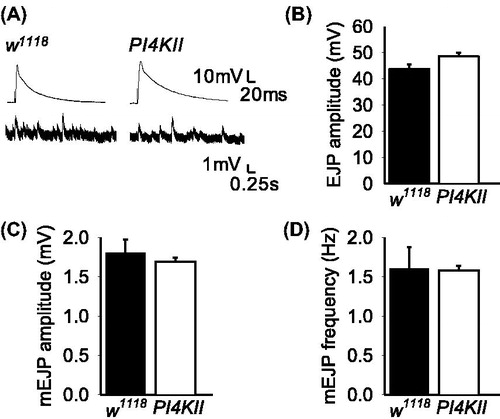
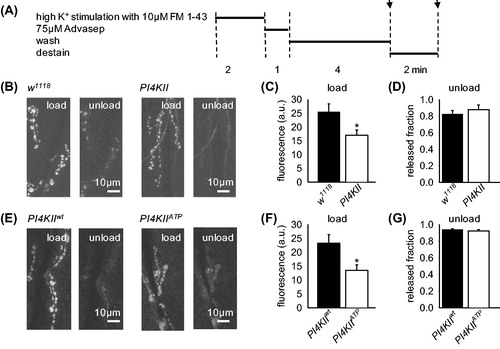
Figure 3. SV recycling is impaired in PI4KII null mutants. (A) Experimental protocol indicating time at which images were captured (indicated by arrows). (B, E) Representative images of presynaptic terminals loaded with FM1-43 during high K+ for 2 min and subsequently unloaded with high K+ stimulation. Preparations were then washed in 0 mM Ca2+ HL6 (with 75 μM Advasep-7 for first minute) for 5 min to remove extracellular FM1-43 and fluorescence was measured. Then high K+ saline was reapplied for 2 min to cause unloading and fluorescence measured again (unload). (C) PI4KII null mutants took up significantly less FM1-43 than controls (n = 7; p < .05), demonstrating impaired vesicle cycling. (D) A similar fraction of FM1-43 was released in controls and PI4KII null mutants, demonstrating that exocytosis was not impaired (p > .05). (F) Larvae expressing PI4KIIATP in a PI4KII null mutant background took up less FM1-43 than larvae expressing PI4KIIwt in a PI4KII null mutant background (n = 6; p < .05). (G) A similar fraction of FM1-43 was released by larvae expressing either PI4KIIATP or PI4KIIwt in a PI4KII null mutant background, demonstrating that exocytosis was not impaired (p > .05). Fluorescence (F) was reported with background F subtracted. Error bars represent SEM.