Figures & data
Figure 1. Kisser probes mimic the subcellular localization of endogenous proteins. (A) Schematic representation of kisser probes described in the manuscript. The fluorescent protein-based kisser probe (FP kisser) encodes two intracellular fluorescent proteins (FP1 and FP2) SE-pHluorin fused to mCherry (pHerry), while the fluorogen-activating peptide (FAP)-based probe (FAP-kisser) encodes an extracellular FAPtag. Kisser probes bind intracellular scaffolds through C-terminal tails homologous to endogenous proteins. Peptide tags (e.g. myc-tag and HA-tag) enable immunodetection. (B) A diagram of Cac-kisser-pHerry, a probe designed to localize with endogenous Cac. (C) A fixed type-Ib bouton where Cac-kisser-pHerry expression is driven pre-synaptically (nSyb-GAL4): Cac-kisser-pHerry (mCherry fluorescence) overlaps with the distribution of endogenous Brp (immunohistochemistry; Cy5). HRP staining (immunohistochemistry; DyLight 405) was used to visualize motor neuron terminals. (D) A diagram of post-synaptic Sh-kisser-FAP, a probe designed to localize with endogenous Sh. The crossed hexagon indicates the fluorogen bound to the FAPtag. (E) A fixed type-Ib bouton where Sh-kisser-FAP expression is driven post-synaptically (24B-GAL4), and the associated fluorogen (Malachite Green; ex. 635 nm/em. 660 nm) labelling. HRP staining (immunohistochemistry; DyLight 405) was used to visualize motor neuron terminals. (F) Same preparation as in E: HA-tag (immunohistochemistry; Cy3) staining was used to detect post-synaptic Sh-kisser-FAP. The probe overlaps in its expression with endogenous Dlg (immunohistochemistry; AF488) and surrounds motor neuron terminals visualized with HRP staining as does the fluorogen (from E).
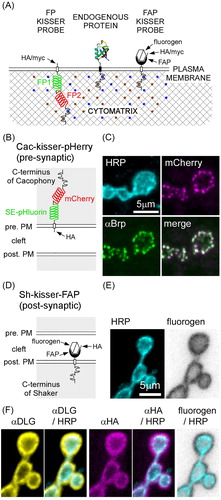
Figure 2. Kisser probes can be used to study the subcellular localization of a protein found in closely apposed membranes of two different cells. (A) A diagram of PMCA-kisser-FAP in the pre-synaptic membrane, a probe designed to localize with endogenous PMCA. The crossed hexagon indicates a fluorogen bound to a FAPtag. (B) A fixed type-Ib bouton where PMCA-kisser-FAP expression is driven in motor neurons (nSyb-GAL4): HRP staining (immunohistochemistry AF488) was used to visualize motor neuron terminals, PMCA-kisser-FAP was stained with Malachite Green. (C) Same preparation as in B: endogenous Brp (immunohistochemistry; AF405), endogenous PMCA (immunohistochemistry; Cy3). Note that endogenous PMCA staining identifies pre- and post-synaptic populations of PMCA, while PMCA-kisser-FAP stained with Malachite Green is pre-synaptic only. (D) A diagram of PMCA-kisser-pHerry in the pre-synaptic membrane. (E) A fixed type-Ib bouton where PMCA-kisser-pHerry expression is driven in motor neurons (nSyb-GAL4): HRP (immunohistochemistry; DyLight 405) staining indicates motor neuron terminals, HA-tag (immunohistochemistry; AF488) was used to detect pre-synaptic PMCA-kisser-pHerry, endogenous PMCA (immunohistochemistry; Cy5). Note that endogenous PMCA staining identifies pre- and post-synaptic populations of PMCA, while HA-tag staining indicates only the pre-synaptic PMCA-kisser-pHerry. Pre-synaptic PMCA-kisser-pHerry shows synaptic and peri-synaptic localization. (F) A diagram PMCA-kisser-pHerry in the post-synaptic membrane. (G) A fixed type-Ib bouton where PMCA-kisser-pHerry expression is driven in the muscle (24B-GAL4): HRP (immunohistochemistry; DyLight 405) staining indicates motor neuron terminals, SE-pH (immunohistochemistry; anti-GFP; AF488) fluorescence indicates the subcellular localization of post-synaptic PMCAkisser-pHerry, endogenous GluRIII (immunohistochemistry; Cy5) indicates the syb-synaptic reticulum. Post-synaptic PMCA-kisser-pHerry has a peri-synaptic localization, partially overlapping with endogenous GluRIII.
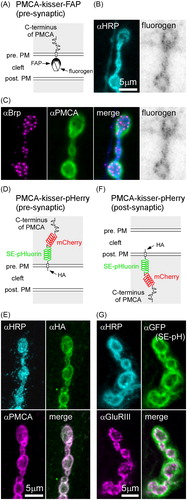
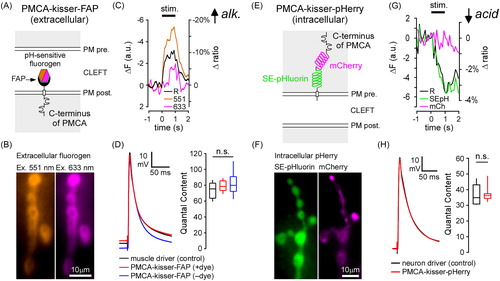
Figure 3. Kisser probes enable functional imaging in protein-defined subdomains of the plasma membrane (PM). (A) A diagram of post-synaptic PMCA-kisser-FAP. The hexagon represents a ratiometric pH-sensitive fluorogen bound to the probe's FAPtag. (B) PMCA-kisser-FAP expressed in the muscle (24B-GAL4), stained with the fluorogen, and visible in the sub-synaptic reticulum of type-Ib boutons. Panels show fluorescence of the fluorogen excited at 551 nm and 633 nm. (C) A 1 s 30 Hz stimulus train causes a decrease (note reversed axes) in fluorogen fluorescence at both wavelengths. A 10% decrease in the 551/633 ratio indicates a transient alkalization of the synaptic cleft. The re-acidification of the synaptic cleft extends beyond 1s post-train cessation. Traincommenced at time = 0. ΔF − change in fluorescence intensity, Δratio − change in ratio of emission wavelength intensities. (D) Untagged post-synaptic PMCA-kisser-FAP and tagged post-synaptic PMCA-kisser-FAP have no detectable impact on neurotransmission: EJP amplitudes are identical to driver control and quantal content is similar as well. (E) A diagram of pre-synaptic PMCA-kisser-pHerry. (F) PMCA-kisser-pHerry expressed in motor neurons and visible in type-Ib boutons (nSyb-GAL4): SE-pHluorin is pH-sensitive; mCherry is a ratiometric control. (G) A 1 s 30 Hz stimulus train causes a decrease in SE-pHluorin fluorescence with little change in mCherry fluorescence. A 4% decrease in the SEpH/mCherry ratio indicates a transient acidification of the pre-synaptic cytosol. The re-alkalization of the pre-synaptic cytosol extends beyond 1 s post-train cessation and is much slower than the corresponding cleft re-acidification. The stimulus commenced at time = 0. ΔF − change in fluorescence intensity, Δratio − change in the ratio of SEpH/mCherry emission intensities. (H) Pre-synaptic PMCA-kisser-pHerry has no detectable impact on neurotransmission: EJP amplitude is identical to driver control and quantal content is similar as well. (D and H) Box plots show first and third quartiles; error bars represent standard deviation.