Figures & data
Figure 1. Summary of signalling interactions between pro- and anti-synaptogenesis pathways in Drosophila. Activating and repressing interactions are marked by ↑ and т symbols, respectively. Cross interactions between members of both pathways sustain the balanced equilibrium of signalling, hence the use of the yin-yang symbol. The actual number of synapses established by a neuron over its target should result from the signalling output determined by the functional status of both cells. Note the similar nature of signalling members in both pathways; for example: small GTPases (Ras85D and 64B), or MAPKs (Wnd, Hep and p38, Lic), etc. The acronyms for the corresponding vertebrate homologues are shown in parenthesis. Original data are from Ferrús’s lab.
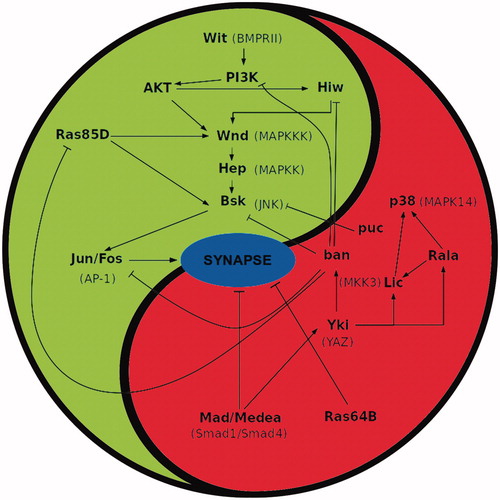
Figure 2. Assembly of synapse components. (A) Synapse in a larval neuromuscular junction. Note the electron dense T bar shaped presynaptic specialization around which synaptic vesicles (asterisk) accumulate. Two small arrows indicate the pre- and postsynaptic cell membranes. (B) Human cortex synapse. Note the pre- and post-synaptic electron-dense specializations. Bar in A = 0.2 and 0.6 µm in B. (C) Sequential assembly of synapse components. Liprin-α (yellow spheres) cluster on the presynaptic membrane followed by Ca2+ channels (blue barrels). Finally, the BRP protein forms a bouquet centred in the active zone attracting the synaptic vesicles (SV, grey spheres) through its Nc82 epitope (green dots). Meanwhile, the postsynaptic membrane accumulates GluRIIA (green barrels) and, later, GluRIIB (red barrels) receptors. (D) The register between the pre- and postsynaptic components is established by the interaction between the presynaptic Neurexin-1 (Nrx-1) and the postsynaptic Neuroliguin1 (Nrl1). Syndecan-1 (Syd-1) is thought to hold the BRP bouquet in place while the peripheral limits of the synapse are determined by the Syd-1 antagonist Spinophilin (Spn). (E) Motor neuron bouton showing several synapses immunolabelled for BRP (red) and GluRIIC (green) showing the perfect register between the pre- and postsynaptic components. Original data are from Ferrus’s lab (A and E); De Felipe’s lab (B); Sigrist’s lab modified from Fouquet et al. (Citation2009) (C) and Muhammad et al. (Citation2015) (D).
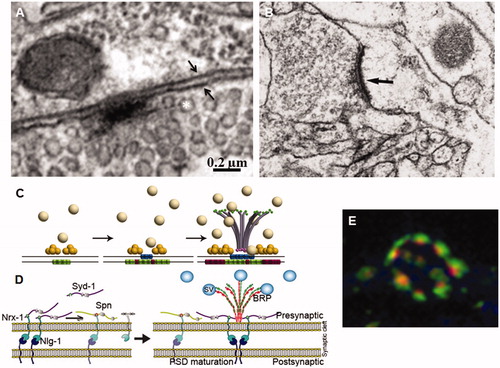
Figure 3. State-Clock model of synaptic changes. As proposed (Frank and Cantera, Citation2014), the biological clocks would drive the 24 hr rhythms in synaptic plasticity. The experience dependent changes in synapses would occur during the activity phase and be consolidated during the inactive phase, but the direction of these changes would not be fixed. In mammals, oscillations in the hypothalamic-pituitary axis (HPA) activity would increase global cortical synaptic activity.