Figures & data
Figure 1. Dorsal D motor neurons undergo synaptic remodeling during early development. (A) (Left) The newly hatched L1 larva contains 3 classes of ventral cord motor neurons: DA, DB, DD. (Right) Five additional postembryonic motor neuron classes (VA, VB, VC, VC, AS) are added to the ventral cord during the L1 to L2 larval transition. (B) During the first larval stage (L1), DD motor neurons (black) provide output to body muscles at ventral presynaptic boutons (purple) and receive input from cholinergic DA/DB neurons (gray) through ACR-12 nACh receptors at postsynaptic terminals (green) on the dorsal side. Arrowhead points to commissure. (C) (Top) DD motor neurons (black) remodel to place presynaptic boutons (purple) on the dorsal side, and relocate postsynaptic terminals (green) to the ventral side for cholinergic input from VA/VB motor neurons (gray). Arrowhead points to commissure. D. DD presynaptic boutons labeled with mCherry::RAB-3 and DD postsynaptic terminals marked with ACR-12::GFP before in early L1 (Left) and after (Right) remodeling at L4 stage. Asterisk labels cell bodies. Scale bar = 10 µm. Images adapted from (He et al., Citation2015). (E) DD neurons remodel over a 4-6-hour period that spans the transition from the L1 to L2 larval stages (yellow).
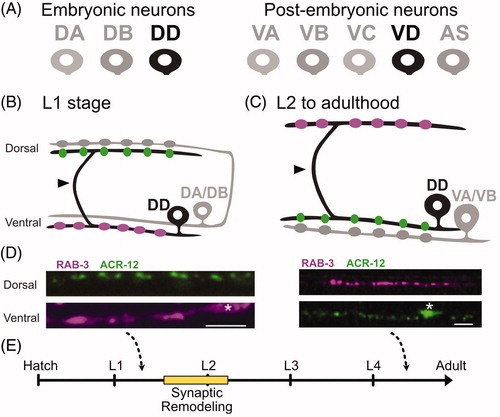
Figure 2. Ventral D (VD) motor neurons ectopically remodel in unc-55 mutants. (A) In adult wild-type worms, DD (black) presynaptic boutons (magenta) are located on the dorsal side and postsynaptic terminals (green) are positioned on the ventral side. VD (gray) presynaptic terminals are positioned on the ventral side (magenta) whereas postsynaptic terminals are located on the dorsal side. (B) In adult unc-55(0) mutants, both DD (black) and VD (gray) presynaptic boutons (magenta) are located on the dorsal side. Postsynaptic terminals (green) of both DD and VD neurons are positioned on the ventral side. (C) (Left) Wild-type worms show robust miniature Post Synaptic Currents (mPSCs) in ventral muscles whereas mPSCs are not detected in unc-55 mutants (Right). Adapted from (Petersen et al., Citation2011). (D) Head touch (asterisk) evokes backward movement in the wild type (top) but unc-55 mutants coil ventrally with head touch (asterisk) due to absence of inhibitory GABAergic input on the ventral side. Scale bar = 250 µm. Adapted from (Petersen et al., Citation2011). (E) The UNC-55/COUP-TF transcription factor functions in VD motor neurons to antagonize expression of synaptic remodeling genes.
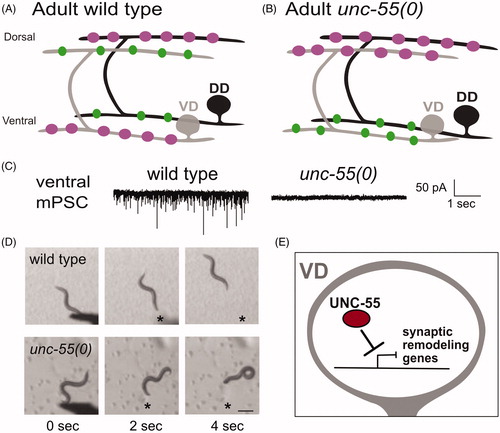
Figure 3. Transcriptional regulation of synaptic remodeling in D-type GABAergic motor neurons. (A) (Left) Morphology of DD motor neuron. (Right) Transcription factors IRX-1/Iroquois MYRF-1/MYRF-2 and HBL-1/Hunchback promote expression of DD remodeling genes, whereas LIN-14 antagonizes remodeling genes. (B) (Left) Morphology of VD motor neuron. (Right) The transcription factor UNC-55/COUP-TFII inhibits expression of IRX-1/Iroquois, HBL-1/Hunchback and MYRF-1/MYRF-2 in VD neurons to prevent ectopic synaptic remodeling.
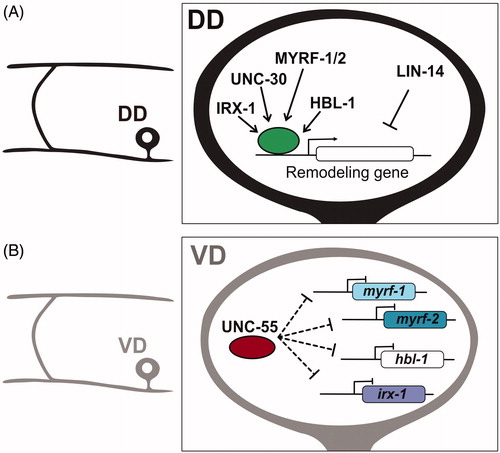
Figure 4. Neuronal activity promotes synaptic remodeling. (A) In DD neurons, neuronal activity promotes the disassembly of ventral presynaptic boutons as well as the formation of new terminals on the dorsal side. (B) Neuronal activity promotes expression of the pro-remodeling transcription factor HBL-1/Hunchback in DD neurons. The homeodomain transcription factor IRX-1/Iroquois/factor drives expression of the DEG/ENaC channel subunit UNC-8. (C) The transcriptional repressor UNC-55/COUP-TFII functions in VD neurons to block expression of the pro-remodeling transcription factor IRX-1/Iroquois and its downstream target UNC-8. (D) Proposed activity-dependent mechanism for synaptic remodeling (1) With depolarization, Voltage-gated Calcium-channels (VGCC) import Ca++ to (2) activate the Ca++-dependent phosphatase Calcineurin/CaN (3) CaN functions upstream of ENaC/UNC-8, which mediates Na++ import, leading to further membrane depolarization and activation of VGCC. These signaling events trigger a positive feedback loop that elevates intracellular Ca++ for activation (4) of a cell-death pathway involving CED-4 and potentially additional downstream effectors (5) of presynaptic disassembly. (E) Presynaptic proteins Endophilin/UNC-57, Rab3, RIM/UNC-10, Munc13/UNC-13, Synaptobrevin/SNB-1, ELKS and Liprin-alpha/SYD-2 regulate synaptic vesicle fusion and neurotransmitter release. (F) The transcription factor IRX-1/Iroquois drives presynaptic disassembly by promoting expression of UNC-8/ENaC expression and an unknown target (X) to remove Endophilin, Rab3, Synaptobrevin and Liprin-α in an activity-dependent mechanism. IRX-1 also drives the elimination of active zone proteins ELKS and Munc13 that are not disassembled by the UNC-8 pathway.
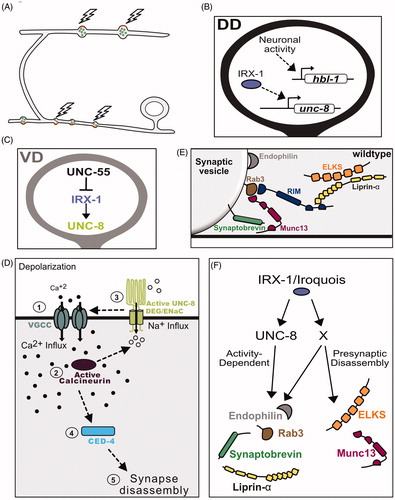
Figure 5. cAMP promotes synaptic remodeling. (A) Transcriptional control of biosynthetic (acy-1/adenylate cyclase) and metabolic (pde-4/phosphodiesterase) regulators of cAMP in DD neurons by IRX-1/Iroquois, UNC-30/PITX and LIN-14. (B) In VD neurons, UNC-30/PITX and UNC-55/COUP-TF promote expression of pde-4/phosphodiesterase and antagonize expression of acy-1/adenylate cyclase to prevent cAMP levels from exceeding a critical threshold that triggers presynaptic remodeling. (C) cAMP promotes the elimination of ventral presynaptic vesicles (green) and the localization of dorsal synaptic vesicles (green) adjacent to clusters of the postsynaptic UNC-49 GABAergic receptors (blue) in dorsal muscles in remodeling DD neurons. cAMP levels are reduced by PDE-4/phosphodiesterase and elevated by the ACY-1/adenylate cyclase and OIG-1/One-Ig-domain transmembrane protein.
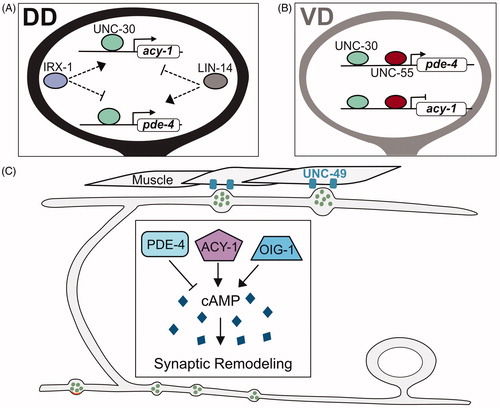
Figure 6. Cellular regulators of synaptic remodeling. (A) Anterograde transport of synaptic vesicles by motor proteins Kinesin1/UNC-116 and Kinesin3/UNC-104 on microtubules (blue) to the anterior distal tip of the dorsal DD neurite is opposed by the retrograde motor complex of Dynein/DHC-1 and Dynactin/DNC-4 that relocates synaptic vesicles to the posterior DD neurite (blue). (B) Experiments with photoconverted Dendra2::RAB-3 demonstrated that RAB-3 from old synaptic terminals (magenta) can be relocated to new dorsal synapses in remodeling DD neurons. (C) Kinesin1/UNC-116 transports synaptic vesicles along microtubules (blue) in the DD commissure. (D) Stable microtubules (blue), intermediate filaments (brown) and the kinase TTBK-3 antagonize synaptic remodeling in tba-1(gf);dlk-1 double mutants (see text). (E) Cell-death pathway components, (EGL-1, CED-4, CED-3) associate with presynaptic mitochondria (yellow) to drive elimination of ventral synaptic terminals. (F) DLK-1 signaling promotes microtubule (blue) dynamics for synaptic remodeling.
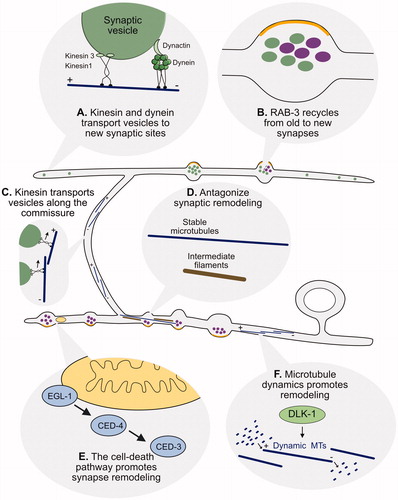
Figure 7. Postsynaptic remodeling. (A) The One-Ig-domain protein, OIG-1, is upregulated by the PITX/UNC-30 transcription factor in early L1 larval DD neurons but turned off by Iroquois/IRX-1 during the late L1 to prevent OIG-1 from antagonizing synaptic remodeling. (B) Graphical representation of dendritic spines protruding from the ventral postsynaptic neurite of a DD neuron and contacting presynaptic terminals of cholinergic VA/VB neurons. Inset shows a fluorescent image of the actin marker, LifeAct::mCherry (magenta), and the postsynaptic protein, LEV-10 (green) at the spine tip. Scale bar = 200 nm. Adapted from (Cuentas-Condori et al., Citation2019) (C) Fluorescent image shows DD dendritic spines (magenta) projecting toward a presynaptic VA neuron (blue). Arrowheads denote sites of contact between postsynaptic spines and the VA process. Scale bar = 1 µm. Adapted from (Cuentas-Condori et al., Citation2019).
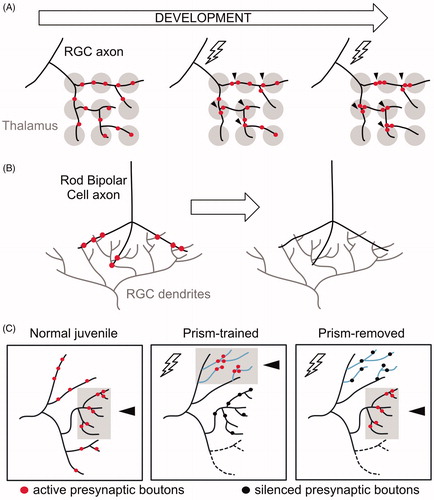
Figure 8. Presynaptic remodeling within intact axons in vertebrate circuits. (A) Initially, a Retinal Ganglion Cell (RGC) axon (black) innervates broadly multiple geniculate neurons in the thalamus (gray). Activity (lightning bolt) induces the relocation and clustering of RGC axons at proximal positions. (B) RGC dendrites (gray) receive input from Rod Bipolar cell axons (black). During early development, synaptic boutons (red) in Rod Bipolar Cells are eliminated while intact axonal trajectories are maintained. (C) Activity drives clustering of presynaptic boutons in the auditory circuit of barn owls. Normal (untrained) juveniles associate visual and auditory cues in the normal axonal region (gray box, arrowhead) where active synaptic boutons cluster (red). Prism-trained owls learn to associate auditory cues with an optically imposed object location. In this paradigm, active synaptic boutons (red) cluster in the adaptive region (gray box, arrowhead) whereas inactive boutons (black) remain in the normal region. After prisms are removed, active synaptic boutons (red) cluster at the normal region (gray box, arrowhead), whereas inactive boutons (black) remain in the adaptive zone.