Figures & data
Fig. 1 Bacterial colonies adjacent to the normal gut epithelium of baboon (A–C), human (D), and rat (E) as observed by transmission electron microscopy. Biopsies from normal animals and from humans were fixed with glutardehyde, osmium, and uranyl acetate; embedded in epoxy resin; ultrathin sectioned; post-stained with uranyl acetate and lead citrate; and examined using an electron microscope. Both small, slender rod-shaped and large, ovoid, and thick rod-shaped bacteria were observed in the cecum of the baboon (A–C), whereas only larger bacteria were observed in the appendix of the human and the cecum of the rat. A matrix, or amorphous material surrounding the bacteria, was identified in all fields. Results are representative of 2 baboons, 2 humans, and 2 rats. The bar represents 2 μm.
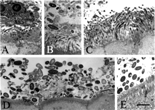
Fig. 2 Bacterial colonies adjacent to the normal rat and human gut epithelium. Rat colon (A–F) or human appendix (G) was flushed with OCT preservative, Flash-frozen, cut, stained with acridine orange and examined by fluorescent microscopy. Longitudinal (parallel to flow of bowel contents) sections (A–D) and transverse (perpendicular to flow of bowel contents) sections (E and F) were cut. Longitudinal sections of the ascending colon (A and B) and the transverse colon (C and D) are shown at 2 different magnifications. Transverse sections of the ascending colon (E) and transverse colon (F) are also shown. The human appendix (G) was sectioned longitudinally. Results are representative of 9 rats and 5 humans. The bars represent 30 μm (A, C, and G) and 15 μm (B and D–F).
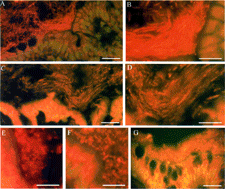
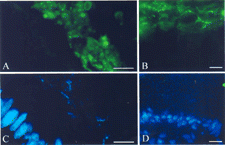
Fig. 3 Association of IgA with biofilms adjacent gut epithelium. Samples were submerged in OCT, flash-frozen, sectioned, and stained with goat anti-rat IgA or goat anti-human IgA followed by a fluorescein labeled anti-goat IgG. Sections were counterstained with the nucleic acid-specific stain DAPI. The presence of IgA (indicated by green fluorescence, A and B) and nucleic acid (indicated by blue fluorescence, C and D) was evaluated by using a fluorescence microscope. One section from the rat cecum (A and C) and one section from the human appendix (B and D) are shown. The portion of the sections photographed was kept constant so that the regions in (A) and (B) correspond exactly to the regions in (C) and (D), respectively. Biofilms were characterized by heavy staining for IgA (green fluorescence) and moderate staining for nucleic acid (blue fluorescence), whereas the nuclei of the gut epithelium were characterized by heavy staining for nucleic acid but not for IgA. The bar represents 15 μm.