Figures & data
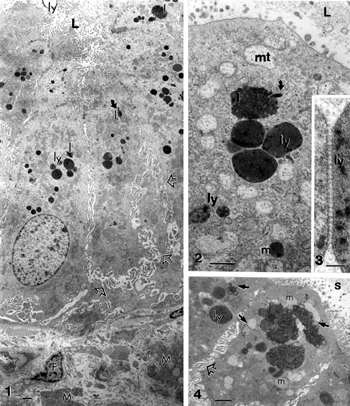
Figures 1–4 Gallbladder epithelium areas from a chronic cholecystitis. Cell crowding and subcellular alterations are blunted microvilli, exaggerated basal and basolateral spaces (open arrows). Apical electron-dense deposits include angulated lysosomes (ly; Figures 2 and 3), heterogenous lipids (l) with paracystalline deposits (curved arrows, in Figures 1 and 2), and altered mitochondria (mt) and fragmented endoplasmic reticulum (ER) in the subapical regions. A detailed view of an intelysosomal junction is illustrated in Figure 3. Apical regions of adjacent cholecytocytes show abundant mucous vesicles (m), heterogenous lipid bodies (l), lysosomes (ly), and muco-liposomes (arrow in Figure 4). The lumen (L) contains biliary sludge (s). Scale bars in Figures 1,2 and 4 equals 1 μm;, in Figure 3 the bar equals 100 nm.
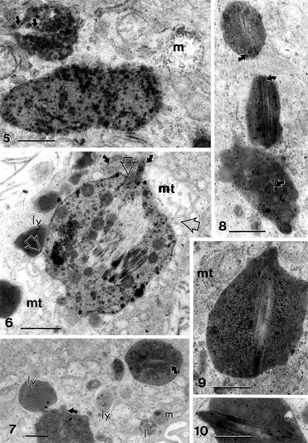
Figures 5–10 Gallery of intracellular, complex lipid inclusions in cholecystitic epithelial cells containing small to large needle-like uncontrasted structures (curved arrows). Open arrows in Figure 6 show associated edges of mitochondria (mt) with lipid complex; m, mucous vesicle; mt, mitochondrion. Scale bar equals 1 μm for all micrographs.
Figures 11 and 12 Needle-like structures in heterogenous lipid deposits (I) (insert). Notice the innumerable adjacent vesicles, altered mitochondrial (mt) fragmented rough ER and polyribosomes. In Figure 12, a network-like array is found at the edge of the main lipidic deposit (pair of small arrows, similar to those found in expelled material in gallstone induction experiments, in Figure 7g of Karkare et al. [Citation[29]]); ly, lysosome; mt, mitochondrion. Scale bars equals to 1 μm.
![Figures 11 and 12 Needle-like structures in heterogenous lipid deposits (I) (insert). Notice the innumerable adjacent vesicles, altered mitochondrial (mt) fragmented rough ER and polyribosomes. In Figure 12, a network-like array is found at the edge of the main lipidic deposit (pair of small arrows, similar to those found in expelled material in gallstone induction experiments, in Figure 7g of Karkare et al. [Citation[29]]); ly, lysosome; mt, mitochondrion. Scale bars equals to 1 μm.](/cms/asset/b1fe4f21-1369-45d9-9633-309499432cc1/iusp_a_19842_f0003_b.gif)
Figures 13–15 Apical region of cholecystitic gallbladder epithelial cell (Figure 13) containing normal mucous vesicles and an heterogeneous lipidic deposit is bisected by a long, curved (Figures 13 and 14) and a thick (Figure 15) needle-like deposit (curved arrow and straight arrows) amidst mitochondria clusters, smooth and rough ER pieces C, centriolar basal body; L, lumen; m, mucous vesicle; mt, mitochondria. All scale bars equal 1 μm.
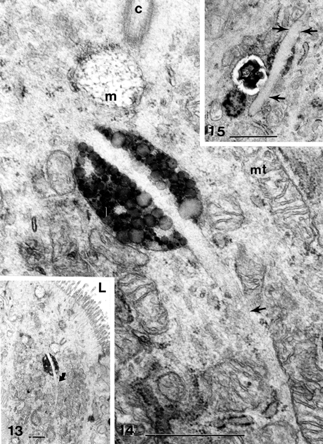
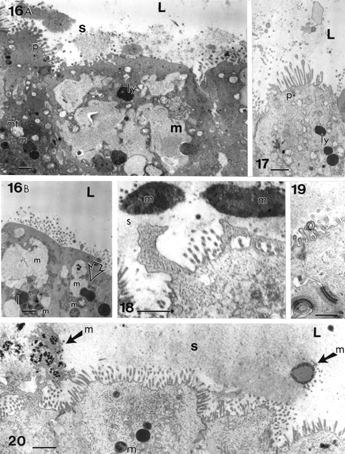
Figures 16–20 Apical region of cholecystocytes coated with pedunculated microvilli (p) and filled with isolated lipid (I), Iysosomal bodies (ly), large, poorly contrasted mucous vesicles (m) that can fuse (Figure 16A, B) and be part of the biliary sludge (s). In Figure 16B, a muco-liposome is marked at the open arrow. Cell junctions are preserved (Figure 19). The heterogeneity of mucos vesicles is shown (Figures 16A–20) after expulsion (Figures 18 and 20, solid arrow with “m” on right) and disintegration into fine and osmiophilic components (“m” marked with oblique arrows on left in Figure 20). L, lumen. In Figure 18: irregular cell apices with secreted anionic mucous; Figure 19 and 20 illustrate apical cell junctions. Scale bars equal 1 μm.
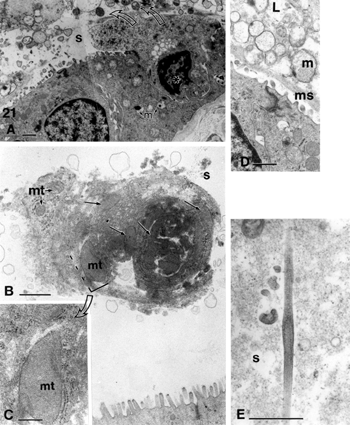
Figures 21 Biliary sludge components from cholecystocyte extrusions (curved arrows) and secretions. Remants of cells found in sludge are cell debris (A, B) wherein mitochondria (mt) membrane and endoplasmic remnants, mucus and intact mucous vesicles (in D), as well as needle-like formation (E) can be identified. L, lumen; m, mucus; ms, mucous sludge; mt, mitochondrium; s, biliary sludge. Scale bars equal 1 μm in A, B, D, and E and 200 nm in c.
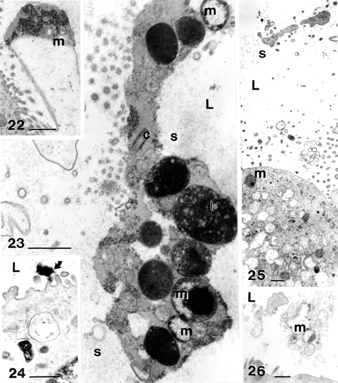
Figures 22–26 Examples of biliary sludge components: cholecystocyte extrusions and degradation material found in the gallbladder lumen (L). Heterogeneous mucus (m) is expelled in the form of exocytosed material (Figure 22) or as almost intact vesicles (Figure 26) if cholecystocytes were sloughed away. Other materials can be pieces of cells (Figures 23 and 24) and electron densely contrasted materials(Fig. 24). C, centriolar body; l, complex lipid. All the scale bars equal 1 μm.