Figures & data
Figure 1. Experiments performed on 4 groups of 2 mice excepted for ODS 48 h which included 3 mice. Normonatremic mice (NN) from group 1 were sacrificed at day 0 (arrow) while uncorrected hyponatremic mice (HN) were sacrificed 4 days after the induction of hyponatremia (arrow). ODS mice were sacrificed as groups 3 and 4, at respectively 12 and 48 h post correction (arrows)
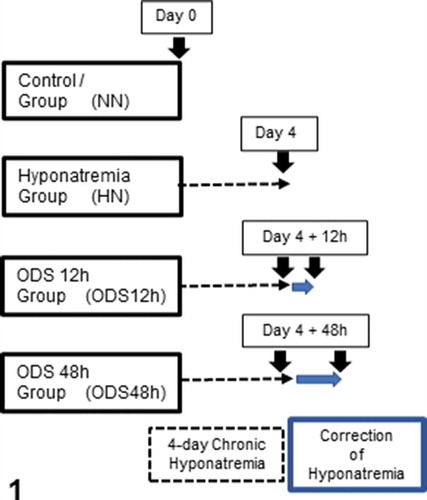
Figure 2. Pane from sagittal sections of normatremia (NN) or Sham, hyponatremia (HN), 12 h after correction of hyponatremia (ODS12h) and 48 h after correction of hyponatremia (ODS48h) mice brains. All stained with hemalum and eriochrome cyanine and ODS 48 h best revealed thalamus (th) as demyelination zone
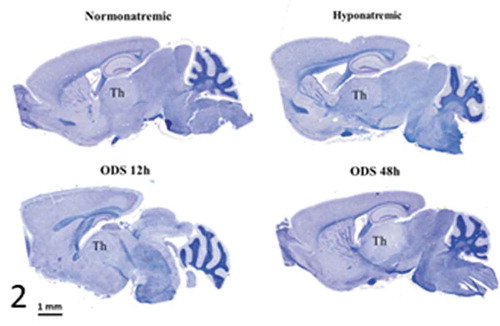
Figure 3. NeuN immunolabeled paraffin sections of NN-, HN-, ODS12h- and ODS48h-treated thalamic ventral posterior nucleus. Scales in ODS48h main micrograph is for all micrographs
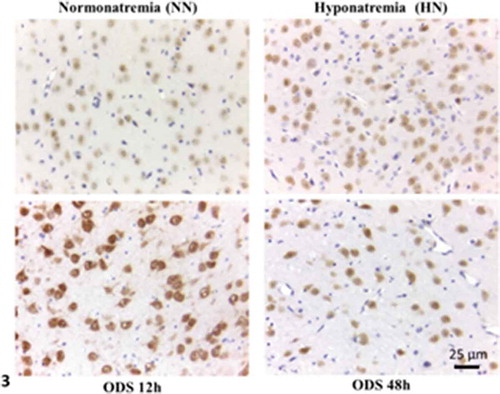
Figure 4. Pane illustrating 1 μm-thick epoxy thick sections of NN, HN, ODS12h and ODS48h from the ventral posterior nucleus thalamic region. Examples of some neuron cell bodies (N), astrocytes (A), oligodendrocytes (O), and myelinated tracts (M) are indicated throughout; in ODS12h and 48 h sections, myelinolysis neuropil cavities are marked (*) while other clear spaces, lined by endothelial cells are small blood vessels. Scales equal 10 μm
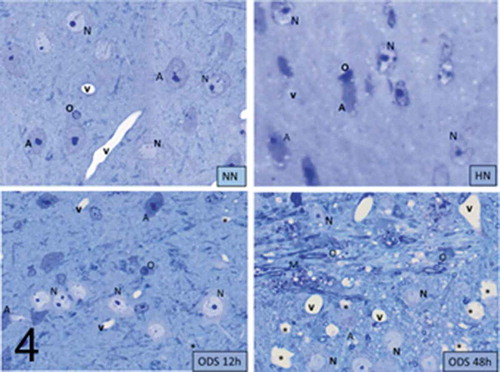
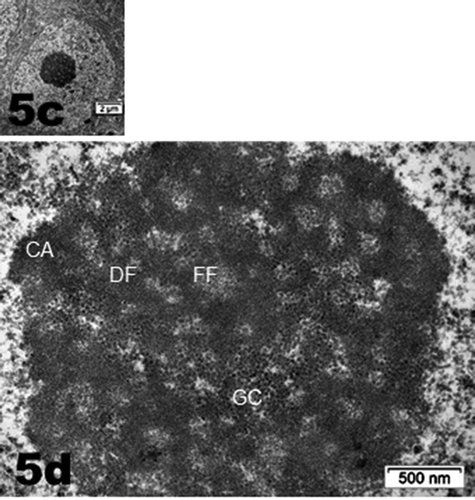
Figure 5. (a–d): Selected views of NN murine neuron cell bodies of the latero-ventral thalamic nuclei and enlarged aspects of nucleoli. (a, c and d): Euchromatic nuclei with slightly indented envelopes with quasi centrally-located nucleoli with their 3 main highly active transcriptional featured components: CA: chromatin associated, part of (c): chromatin, as associated with the inner nuclear envelope; DF: dense fibrillar, FF: fine fibrillar region accompanied by its cloud of ribonucleoprotein transcript products as granules i.e. small and large ribosomal and other RNAs (Granular Component as GC). (b) Enlarged Golgi zone of (a)
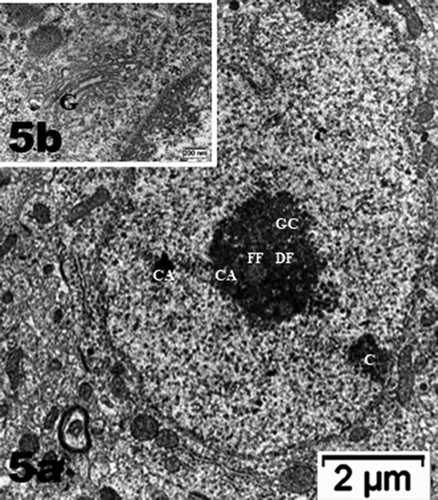
Figure 6. Neuron cell body of NN murine latero-ventral thalamic region. (a): Euchromatic nucleus with deeply indented envelope reaching, in its central zone, the large nucleolus and its 3 main aligned components indicating high transcription activities: CA: chromatin associated, GC: granular center (ribonucleoprotein components); CA+GC both forming nucleolar organizer centers or NORs; DF: dense fibrillar and FF: fine fibrillar region. G: Golgi apparatus; axo-somatic synapses are marked by small white arrows. Compare this micrograph with that of
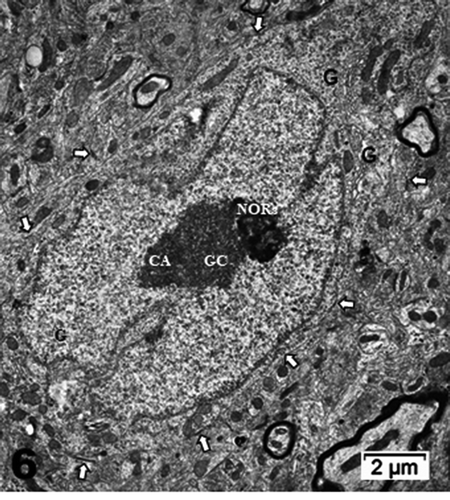
Figure 7. (a–d): HN neuron cell bodies of the parenchyma of the latero-ventral thalamic parenchyma showing the nucleus topology change (a–b) as well as the nucleolus. (a and b) segregation of the nucleolus CA/DF regions from GC component (curved arrows in A and D) suggestive of a reduced or stoppage in ranscriptional activities because accumulated ribonucleoproteins (GC) amassed separated from the chromatin (CA) becoming concentric of GC, and its extension as dense fibrillar (DF). The fine fibrillar region (pale circles in nucleolus depicting transcription (FF) is absent. In the GC mass, interstices are formed (I) as CA leaves the nucleolus. Example of perikaryon of (b) in (c) revealed scattered free polysomes but none attached to adjacent endoplasmic reticulum (white arrow), part of Golgi zone, an endosome (e) and many coated vesicles, maybe forming autophagosomes (ly); a peculiar fuzzy osmiophilic mass (m) deposits among the cytosol. Note discrete intercellular spaces formed by [Na+] depletion
![Figure 7. (a–d): HN neuron cell bodies of the parenchyma of the latero-ventral thalamic parenchyma showing the nucleus topology change (a–b) as well as the nucleolus. (a and b) segregation of the nucleolus CA/DF regions from GC component (curved arrows in A and D) suggestive of a reduced or stoppage in ranscriptional activities because accumulated ribonucleoproteins (GC) amassed separated from the chromatin (CA) becoming concentric of GC, and its extension as dense fibrillar (DF). The fine fibrillar region (pale circles in nucleolus depicting transcription (FF) is absent. In the GC mass, interstices are formed (I) as CA leaves the nucleolus. Example of perikaryon of (b) in (c) revealed scattered free polysomes but none attached to adjacent endoplasmic reticulum (white arrow), part of Golgi zone, an endosome (e) and many coated vesicles, maybe forming autophagosomes (ly); a peculiar fuzzy osmiophilic mass (m) deposits among the cytosol. Note discrete intercellular spaces formed by [Na+] depletion](/cms/asset/eb9ac832-43fa-4016-8f1a-09ca8cf7f773/iusp_a_1853865_f0007_b.gif)
Figure 8. (a–d): ODS12h nerve cell bodies in the demyelinated region, ventrolateral nuclei of the mouse thalamus. Nucleus shows a prominent nucleolus where both chromatin- associated (CA) and granular center (GC) are recognized and completed separated from one another (curved arrow) along with other perikaryal damages (arrows in a–c); Ah: axon hillock. In (d): damages consist in ER membrane’s degradations, including those of the nucleus envelope (arrowed), the endoplasm and Golgi apparatus (G); lipofuscin bodies (lp). CNS parenchyma is the degraded neuropil in (a–b and d)
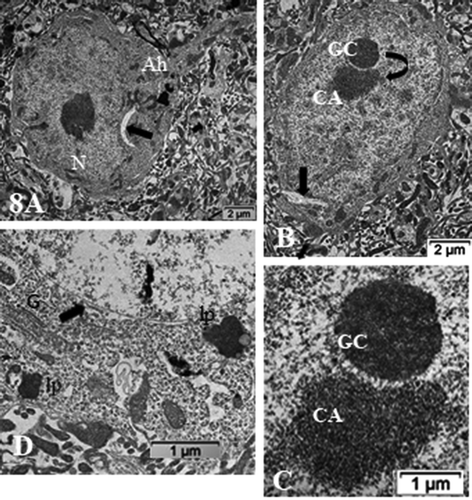
Figure 9. (a–d): Pane with ODS12h nerve cell bodies preserved, adjacent to the demyelinated zone of the ventrolateral thalamus showing typical euchromatic nuclei with prominent nucleoli where components, although evident in all the featured micrographs, do not form NORs and are centrally placed and somewhat segregated into CA and GC regions. In (b and d), highly contrasted oligodendrocyte section parts in the surrounded neuropil (O). In (d), small white arrows display examples of axo-somatic synaptic sectors. Ah: axon hillock; G: Golgi zone; arrow shows autophagosome. Scale in A is the same for (b–d)
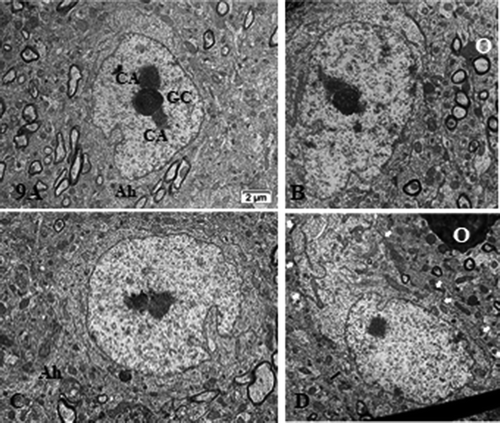
Figure 10. (a–c): TEM pane with ODS 48 h thalamus euchromatic nerve cell body aspects among the neuropil where damages can still be viewed as myelin damages remained throughout. Prominent nerve cell bodies with nucleolus have regained round with indentations and highly contrasted but active nucleolus organization (NORs) have reorganized into all active parts as those described in NN cells. Arrows indicate nucleus envelope damages. Ah: axon hillock
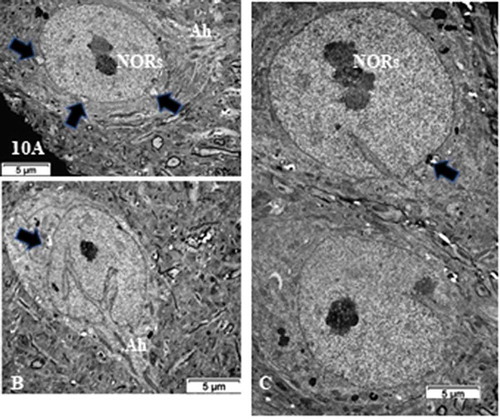
Figure 11. (a,b): Pane of one ODS 48 h nerve cell body of murine thalamus showing similar aspect nucleus as found in some NN cells (see ), including a similar display of its envelope indent. The prominent nucleolus is revealed with NORs and components with a huge cloud of ribonucleoproteins (GC) and the chromatin associated (CA). Golgi apparatus encircles the nucleus in the reactivated perikaryon. Neuropil surroundings still reveal discrete to evident demyelinated axon damages as remnant whorls or evident interfascicular cavities or voids. (b): Enlarged aspect of the (a) nucleus depicting nucleolus where ribonucleoprotein reach the adjacent, envelope indent whose neuroplasm content is loaded by numerous polysomes, aimed at translational activities
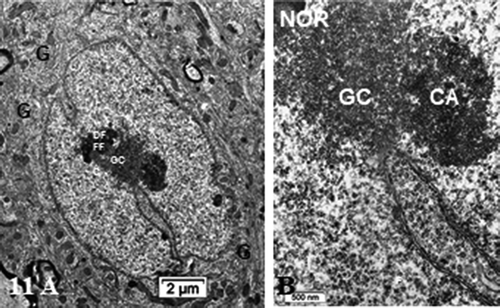
Figure 12. (a–e): Pane of ODS 48 h nerve cell bodies of murine thalamus containing several examples of nucleus profiles (a–d), associated prominent activated nucleoli revealing many NORs, and adjacent neuropil with damaged axons whose removal of myelin have left intercellular spaces or voids (stars in b, d and e). (a) peculiar neuroplasm deposit is arrowed in (a) (see ). (c): enlarged nucleolus with NORs: (g): granular component; CA: chromatin associated chromatin. (e): Neuron adjacent to a myelinated nerve bundle; star: intercellular cavity left from myelinolysis
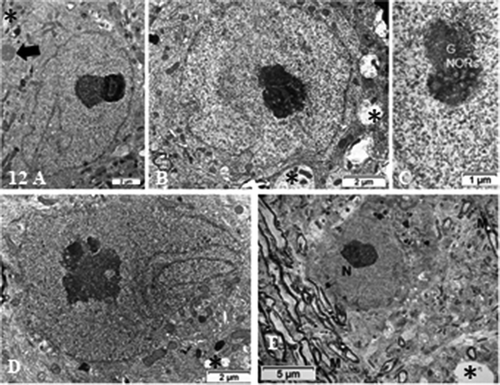
Figure 13. (a–d): TEM of ODS48h ventro-lateral thalamus neuron cell bodies with the enlarged perikaryon. Both depict long deep, twisted indents of the nucleus envelope as if reaching the active nucleolus, as in viewing many organelles i.e. G: Golgi, ly: lysosomes, ER and mitochondria. Ah: Axon hillock region; CA: chromatin associated to nucleolus, GC: granular center; O; oligodendrocyte; Arrow in (d) indicates fragile nucleus envelope endoplasm defects
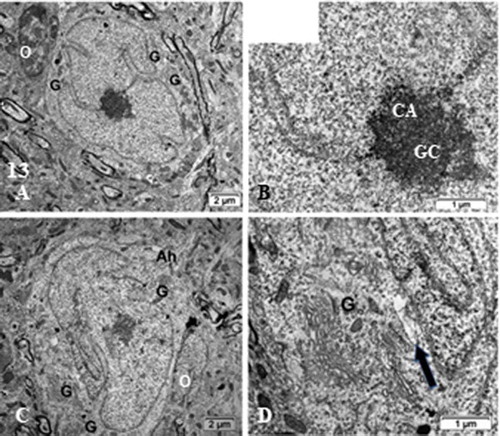
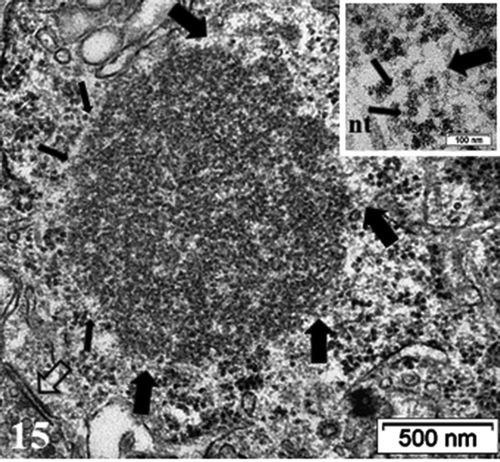
Figure 14. TEM aspect of the enlarged neuron perikaryon of with many organelles i.e. G: Golgi, ly: lysosomes, ER and mitochondria along with a peculiar round fibro-particulate aggregate. N; nucleus. Some of the axo-somatic synapses are indicated by open arrows; G: Golgi, ly: lysosomes, ER and mitochondria along with a peculiar round fibro-particulate aggregate. N; nucleus; axo-somatic synapse (arrowed). As: Astrocyte parts with beta-glycogen granules
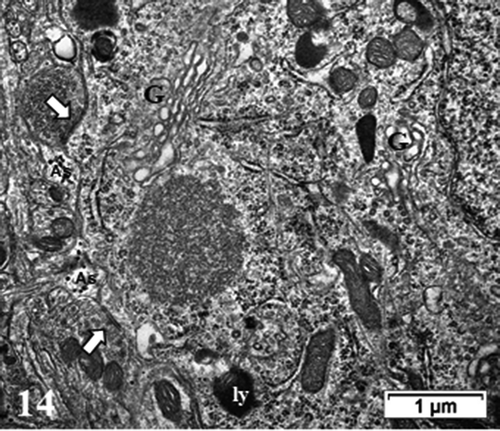
Figure 15. Detailed view of the fibro-particulate aggregate where sticking out filamentous extensions are marked by thick arrows and thin arrows indicate particulate parts. Axo-somatic synapse (open arrow). Insert: Exhibit of the edge of the aggregate somewhat concealed components indicating that threads emerging out of it are from 4–5 nm in thickness (wide arrows) with mRNA – polysome structures (thin arrows); nt: neurotubule