Figures & data
Table 1 Tumors of Fibroblastic and Myofibroblastic Origin in Children and Adolescents
Table 2 Triaging of Fibroblastic and Myofibroblastic Tumors in Children and Adolescents
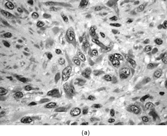
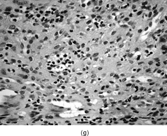
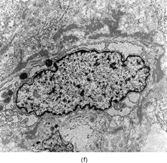
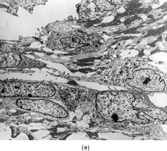
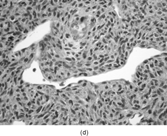
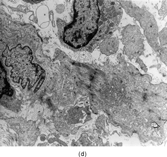
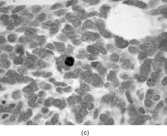
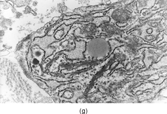
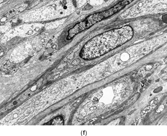
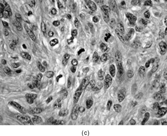
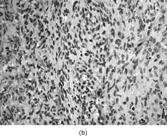
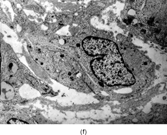
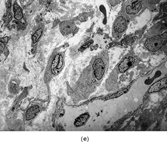
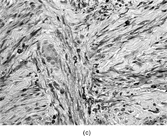
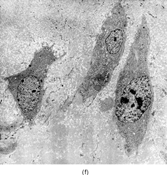
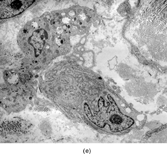
FIG. 1 Infantile fibrosarcoma composed of interlacing fascicles of spindled cells (a, b, original magnification × 100 and 200 × respectively; H & E) with minimal intervening stroma and lack of collagen matrix. The tumor cells have a moderate degree of pleomorphism with frequent mitotic figures (c, original magnification × 400 H & E). Areas within the tumor may show a prominent hemangiopericytoma-like pattern (d, original magnification × 100 H & E). Infantile fibrosarcoma possesses extracellular fibrillogranular material, which is helpful in differentiating this malignant tumor from other fibroblastic/myofibroblastic tumors (e, f, × 3,000 and × 10,000 respectively, transmission electron microscopy). After chemotherapy for a nonresectable infantile fibrosarcoma (g, original magnification × 100 H & E), the spindle cells are conspicuously absent, leaving a fibrous matrix background with a prominent bland vascular pattern.
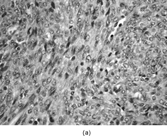
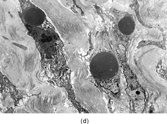
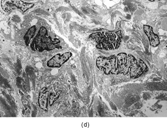
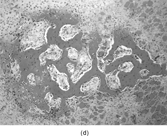
FIG. 2 Infantile rhabdomyofibrosarcoma resembles congenital infantile fibrosarcoma with a highly cellular spindle tumor cell population and frequent mitotic figures (a, b, original magnification × 200 and × 400, respectively). Myogenic immunocytochemical markers may discover rare immunoreactive cells(c, myogenin antibody, original magnification × 400). Ultrastructural examination provides evidence for rhabdomyoblastic differentiation in the spindle cells with detection of thin and thick filaments and z-band material(d, × 4,000, transmission electron microscopy).

Table 3 Tumors of Fibroblastic and Myofibroblastic Origin in Children and Adolescents: Immunocytochemical, Ultrastructural, and Cytogenetic Features
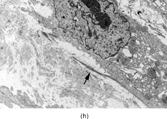
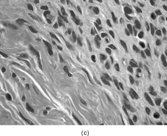
FIG. 3 Myofibromas are often surrounded by a thin fibrous capsule (a, original magnification × 100, H&E) and show increased cellularity at the periphery of the lesion. Toward the central portion of the mass, there is decreased cellularity and increased collagen matrix deposition (b, original magnification × 200, H&E). Mitotic activity is frequently noted with no affect on clinical outcome of the lesion (c, original magnification × 400, H&E). Some myofibromas have a hemangiopericytoma-like pattern (d, original magnification × 100, H&E) with subendothelial proliferation of myofibroblasts. Necrosis with or without dystrophic calcifications is also commonly found (e, original magnification × 200, H&E). Ultrastructural examination reveals spindle cells with myofibroblastic features, including longitudinal filaments (f, original magnification × 4,000), prominent dilated rough endoplasmic reticulum with granular material (g, original magnification × 12,000), and fibronexus structures (arrow, h, original magnification × 10,000).
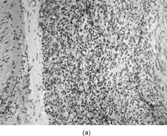
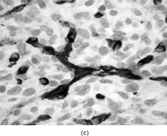
FIG. 4 Pericytoma with a t(7;12) translocation ischaracterized by plump ovoid to spindled cells in arranged around capillary-sized vessels often containing erythrocytes (a, b, original magnification × 100 and × 400, respectively, H&E). The endothelial cells immunoreact with CD34 antibody (c, CD34 antibody, original magnification × 400); however, the tumor cells are nonreactive. The tumor cells express smooth muscle actin (d, smooth muscle actin antibody, original magnification × 400). Ultrastructural examination reveals plump spindle cells in close proximity to thin-walled vessels lined by bland endothelial cells (e, original magnification × 1,200) with abundant extracellular basal-lamina like material adherent to the cell surfaces (f, original magnification × 8,000).

FIG. 5 Inclusion body fibromatosis (congenitalinfantile digital fibromatosis) is composed of bland fibroblastic cells arranged into sheets and fascicles with abundant collagen matrix (a, original magnification × 200, H&E). Trichrome (b, original magnification × 400) and smooth muscle actin (c, original magnification × 200) staining may reveal oval cytoplasmic bodies with the spindle cells. Upon ultrastructural examination, these cytoplasmic bodies prove to be whorls of actin filaments that are characteristic for this specific fibroblastic tumor (d, original magnification × 5,000).

FIG. 6 Fibrous hamartoma of infancy iscomposed of fibrous tissue, primitive mesenchyme within a myxoid matrix and mature adipose tissue (a, original magnification × 100, H&E). There is an abrupt transition from “mature” fibrous tissue to primitive mesenchyme within a myxoid stroma (b, c, original magnification × 200 and × 400, respectively, H&E). The fibroblastic tumor cells with irregular and deeply indented nuclear borders are embedded in a dense haphazardly arranged collagen background (d, original magnification × 3,000, transmission electron microscopy).
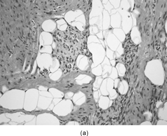
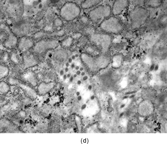
FIG. 7 Juvenile hyaline fibromatosis is composed of bland spindled cells embedded in a dense collagenous matrix lacking a fibrillary character for the most part (a, b, original magnification × 100 and × 200, respectively, H&E). Ultrastructural examination displays the dilated rough endoplasmic reticulum containing granular material (c, original magnification × 3,500) and intracytoplasmic collagen fibers (d, original magnification × 14,000). Often, dilated membrane-bound vesicles with granular material are lined by a thin cell surface membrane and nearly in contact with the extracellular matrix(e, original magnification × 4,500).

FIG. 8 Fibrodysplasia ossificans progressiva is composed of plump spindled cells in a collagen matrix, which varies from myxoid to chondroid (a, original magnification × 400, H&E). The matrix undergoes hyalinization with “entrapment” of the spindled cells (b, original magnification × 400, H&E). The matrix becomes densely eosinophilic and resembles osteoid with ensuing calcification (c, original magnification × 200, H&E). Areas within the lesional tissue form intersecting trabeculae of calcified bone with adjacent chondroid-like areas (d, original magnification × 100, H&E). Ultrastructural examination of the noncalcified matrix and early lesions reveal plump fibroblastic cells with mildly to moderately dilated rough endoplasmic reticulum and collagen matrix (e, original magnification × 3,000). Elongated spindle cells elaborating collagen matrix and embedded in relatively abundant collagen matrix are also noted (f, original magnification × 2,000). Some of the ground substance is clumped together and has an “amorphous” character similar to that seen in osteogenic tissues.