Figures & data
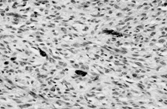
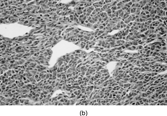
Fig. 1 Dermatofibrosarcoma with uniform spindle cells with scanty cytoplasm, in characteristic storiform pattern.
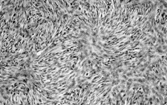
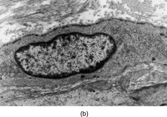
Fig. 2 Dermatofibrosarcoma. Electron micrograph showing spindle cells with scanty cytoplasm containing scanty profiles of rough endoplasmic reticulum and no other specific features.
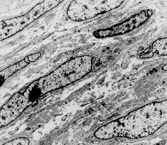
Fig. 3 Myxoid dermatofibrosarcoma. Other parts of this tumor showed more typical features, with CD34 positivity.
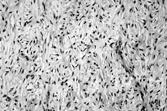
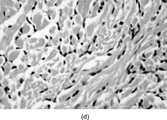
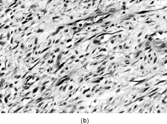
Fig. 5 Fibrosarcoma in dermatofibrosarcoma. Fascicles of spindle cells in herringbone pattern. Cells have inconspicuous cytoplasm and increased mitotic index.

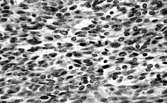
Fig. 6 Giant cell fibroblastoma. There are spindle and multinucleate cells in a fibromyxoid stroma. Similar cells line pseudovascular spaces.
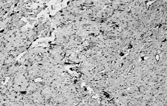
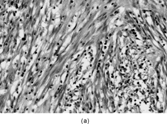
Fig. 7 Solitary fibrous tumor. (a) The tumor has a circumscribed border and a peripheral hemangiopericytic pattern. (b) Fibrous and cellular areas are abruptly juxtaposed. (c) Bland spindle cells are arranged in a patternless pattern in cellular areas. (d) Cords of cells in a collagenous focus.
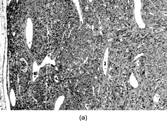
Fig. 8 Solitary fibrous tumor. Cells have ultrastructural features of fibroblasts, with variable amounts of rough endoplasmic reticulum and no external lamina.
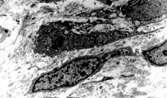
Fig. 10 Pericyte (a) adjacent to blood capillary vessel and (b) enclosed within layers of external lamina, showing pinocytosis, Golgi complex, and abundant cytoplasmic filaments, focally with dense bodies suggesting myoid differentiation.
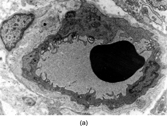
Fig. 11 Hemangiopericytoma. (a) Typical appearance, with prominent and widespread typical vascular pattern and uniform plump cells. This tumor is CD34 negative. (b) malignant hemangiopericytoma is a diagnosis of exclusion; many tumors with this appearance prove to be poorly differentiated synovial sarcomas.
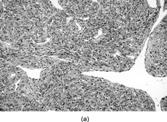
Fig. 12 (a) Myofibroblast showing fibronexus fibrils in continuity with intracytoplasmic “stress fibers.” (b) Stellate myofibroblast from a case of nodular fasciitis, showing peripheral filament bundles and abundant rough endoplasmic reticulum in each of the arms of the cell.
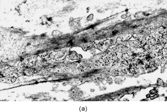
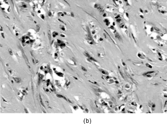
Fig. 13 Low-grade myofibrosarcoma. (a) Moderately cellular tumor with fascicular architecture and scanty lymphocytic infiltrate. (b) Cells are mildly pleomorphic with variable nuclear staining; some have small nucleoli. (c) Myxoid areas resemble nodular fasciitis but differ in displaying nuclear atypia.
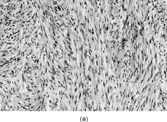
Fig. 14 Ultrastructure of low-grade myofibrosarcoma. This neoplastic cell has peripheral filament bundles and plentiful rough endoplasmic reticulum, and stress fibers with fibronexus.
Table 1 Some Tumors with Hemangiopericytomatous Pattern
Table 2 Immunohistochemistry of Smooth Muscle and Fibro/Myofibroblastic Tumors