Figures & data
Table 1. Results from the morphometric analyses of Spiniferites elongatus sensu lato cysts retrieved from surface sediments and sediment trap sequences.
Table 2. Location and local surface water conditions for the Beaufort Sea surface sediment samples from which cysts of Spiniferites elongatus sensu lato were isolated.
Figure 1. Scatter diagram showing cyst width against cyst length for measured elongate Spiniferites cysts. A, Data for all individual measurements from surface sediments. Also shown are the cyst width and length of the holotype of Spiniferites elongatus (black dot) and the topotype extremes (dashed arrow) (Reid Citation1974), and the average (black Greek cross) and range (black thickened axes) of the cysts measured by Ellegaard et al. (Citation2003), as well as the cyst width and length of the holotype of Spiniferites ellipsoideus (black square) and its type assemblage (grey shaded area) (Matsuoka Citation1983). B, Average values for each regional surface sediment assemblage, with the standard deviation indicated. Also shown are the values from Harland and Sharp (Citation1986) for specimens recovered from the Firth of Forth (UK), and the Norwegian and Barents seas. C, Individual measurements for the specimens recovered from sediment traps in eastern (EHB) and western (WHB) Hudson Bay.
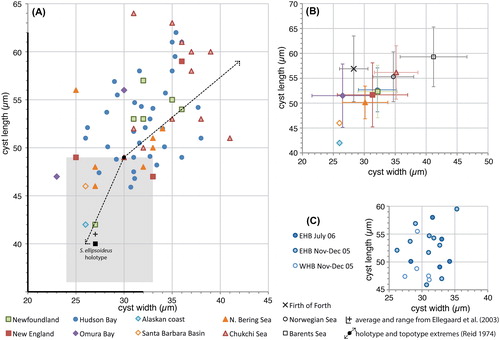
Figure 2. Scatter diagram showing antapical process length against cyst length for measured elongate Spiniferites cysts. A, Data for all individual measurements from surface sediments. The holotype is shown for reference (black dot). B, Average values for each regional surface sediment assemblage, with the standard deviation indicated. Also shown are the values from Harland and Sharp (Citation1986) for specimens recovered from the Firth of Forth (UK), and the Norwegian and Barents seas. Note that the ‘antapical membrane length’ given by these authors (Harland and Sharp Citation1986, their table 1) is considered to correspond the antapical process length as measured here. C, Individual measurements for the specimens recovered from sediment traps in eastern (EHB) and western (WHB) Hudson Bay.
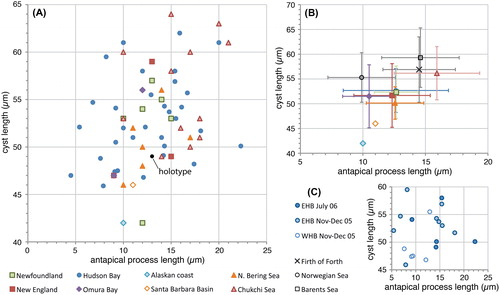
Figure 3. Scatter diagram showing antapical process length against antapical crest height for measured elongate Spiniferites cysts. A, Data for all individual measurements from surface sediments. B, Average values for each regional surface sediment assemblage, with the standard deviation indicated. C, Individual measurements for the specimens recovered from sediment traps in eastern (EHB) and western (WHB) Hudson Bay.
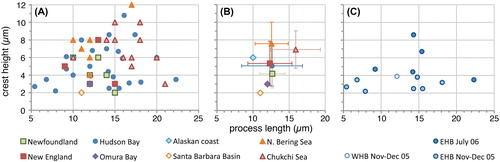
Figure 4. Scatter diagram showing antapical process length against elongateness (i.e. the ratio between cyst length and cyst width) for measured elongate Spiniferites cysts. The holotype is shown for reference (black dot). A, Data for all individual measurements from surface sediments. B, Average values for each regional surface sediment assemblage, with the standard deviation indicated. Also shown are the values from Harland and Sharp (Citation1986) for specimens recovered from the Firth of Forth (UK), and the Norwegian and Barents seas. C, Individual measurements for the specimens recovered from sediment traps in eastern (EHB) and western (WHB) Hudson Bay.
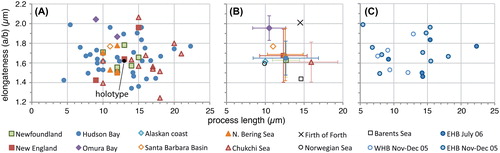
Figure 5. Scatter diagram showing antapical crest height against elongateness (i.e. the ratio between cyst length and cyst width) for measured elongate Spiniferites cysts. A, Data for all individual measurements from surface sediments. B, Average values for each regional surface sediment assemblage, with the standard deviation indicated. C, Individual measurements for the specimens recovered from sediment traps in eastern (EHB) and western (WHB) Hudson Bay.
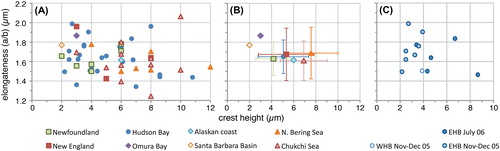
Figure 6. Frequency distribution diagram (A–C: 2 μm bins; D: 1 μm bins) for measured elongate Spiniferites cysts, for the total dataset (blue), cysts from Hudson Bay (red) and cysts from the Chukchi and Bering Sea (green). A) Cyst length; B) Cyst width; C) Antapical process length; D) Antapical crest height.
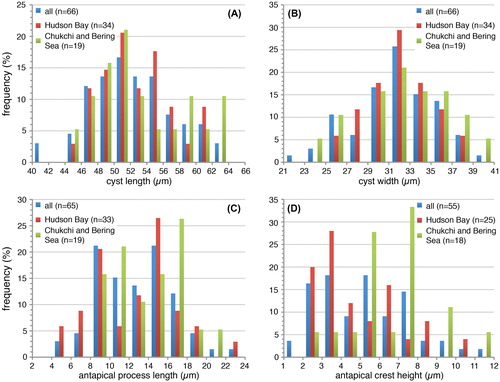
Plate 1. Bright-field photomicrographs of the Spiniferites elongatus sensu lato cysts isolated from the Beaufort Sea that were used in the genetic analyses. The National Center for Biotechnology Information (NCBI) GenBank accession numbers associated to the specimens are KU358942 (cyst 1), KU358943 (cyst 2), KU358944 (cyst 3), KU358945 (cyst 4), KU358946 (cyst 5), KU358947 (cyst 6), KU358948 (cyst 7), KU358949 (cyst 8), and KU358950 (cyst 9). Scale bars = 10 μm.
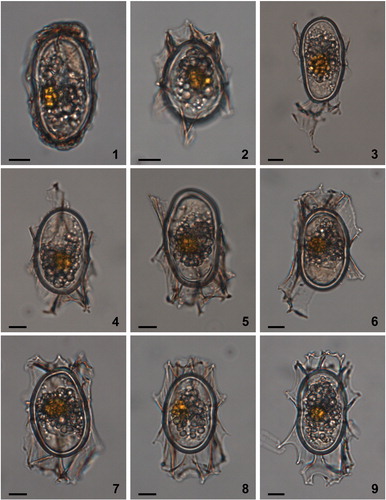
Plate 2. Bright-field photomicrographs of Spiniferites elongatus from off Newfoundland (1–2, 3, 4–5, 6–7) and the Alaskan Coast (8–9). The lines in figure 1 indicate how cyst morphometrics were measured. a = cyst length, b = cyst width, c = antapical process length, d = antapical membrane height. Scale bars = 10 μm.
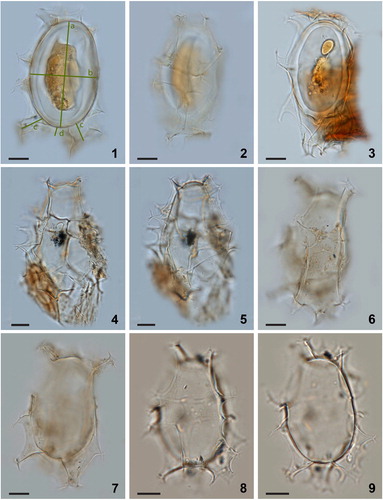
Plate 3. Bright-field photomicrographs of Spiniferites elongatus from Chukchi Sea. 1–3. Dorsal, optical section, and ventral view of a specimen with a high membrane between the two dorsal antapical processes. 4–5. Specimen illustrating the unequal development of the dorsal antapical processes. 6–9. Further illustration of the morphological variability of specimens in a single assemblage. Scale bars = 10 μm.
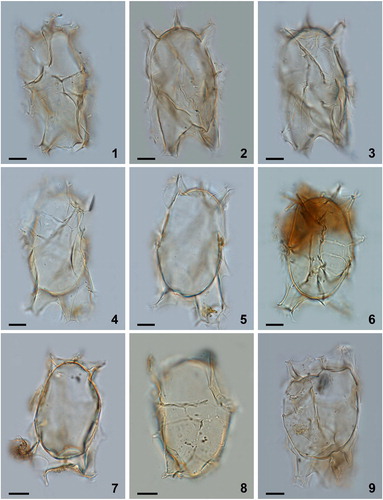
Plate 4. Bright-field photomicrographs of Spiniferites elongatus from (1–6) Hudson Bay surface sediments, (7) eastern Hudson Bay sediment trap deposits collected over July 2006, and (8, 9) western Hudson Bay sediment trap deposits collected from November to December 2005. Note the marked antapical suturocavation in the specimen in figure 7, and the differences in morphology between cysts produced over the same growing season (8 and 9). Figures 1 and 9 are examples of specimens that can be identified as Spiniferites elongatus – Beaufort morphotype. Scale bars = 10 μm.
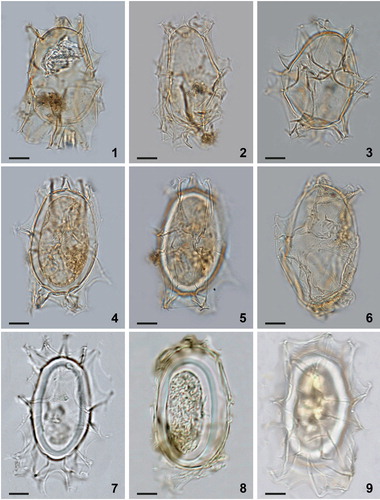
Plate 5. Bright-field photomicrographs of Spiniferites elongatus sensu lato from New England (1, 2, 3), Omura Bay, Japan (4, 5), and the Bering Sea (6–9). While the specimens shown in figures 7–9 are good examples of Spiniferites elongatus – Beaufort morphotype, the specimens shown in figures 4–6 illustrate morphologies somewhat intermediate between typical Spiniferites elongatus and the Beaufort morphotype. Scale bars = 10 μm.
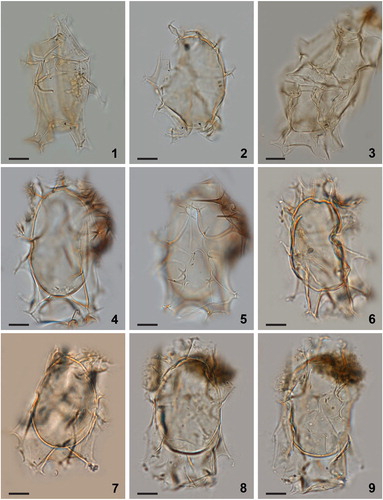
Figure 7. Maximum likelihood (ML) phylogenetic tree based on 799 aligned nucleotides of the nuclear large subunit ribosomal RNA (LSU rDNA) using the TIM3 + G model with Heterocapsa triquetra (Ehrenberg Citation1840) Stein 1883 and Prorocentrum micans as outgroup taxa. Alignment length includes gaps. The numbers at the nodes of the branches indicate the ML bootstrap (left) and Bayesian posterior probability (right) values; only values ≥50% or 0.5 are shown. Genbank accession numbers are provided.
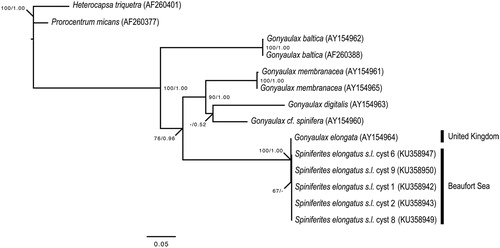
Plate 6. 1–6. Dual interference photomicrographs (1–4) and scanning electron micrographs (5, 6) of Spiniferites elongatus – Norwegian morphotype, from late Pleistocene (Marine Isotope Stage 5e) sediments from the Vøring Plateau, Norwegian Sea. 7–9. Newly produced photomicrographs of the Miocene specimen from Japan that had been designated the holotype of Spiniferites ellipsoideus, herein considered a junior synonym of Spiniferites elongatus. Figures 7–9 courtesy of Kazumi Matsuoka, with kind permission. Scale bars = 10 µm.
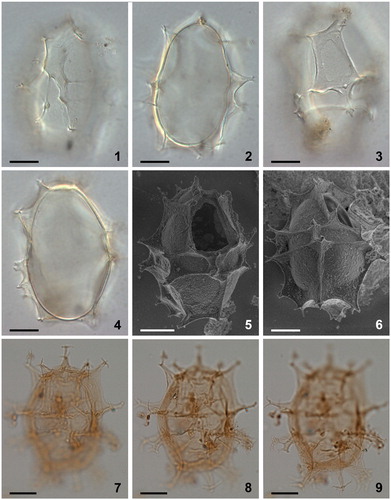
Plate 7. 1–4. Scanning electron micrographs of apical views of four different specimens of Spiniferites elongatus from the Barents Sea with significant variation in the elevation of sutural crests, all showing consistently four apical plates (1′–4′). Note a faint suture between 1′ and 4′. ‘a’ indicates reflection of ventral pore; ‘b’ indicates reflection of apical pore. as = anterior sulcal plate. Scale bars = 10 µm.
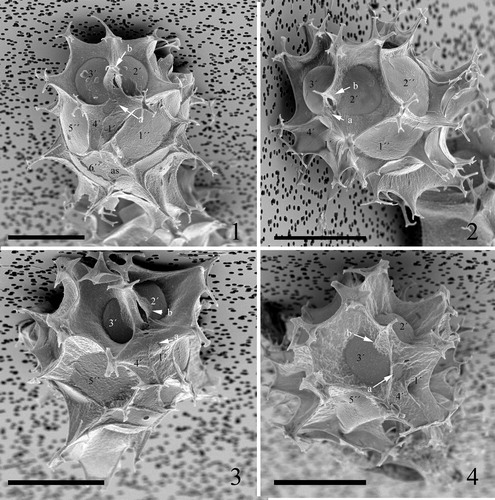
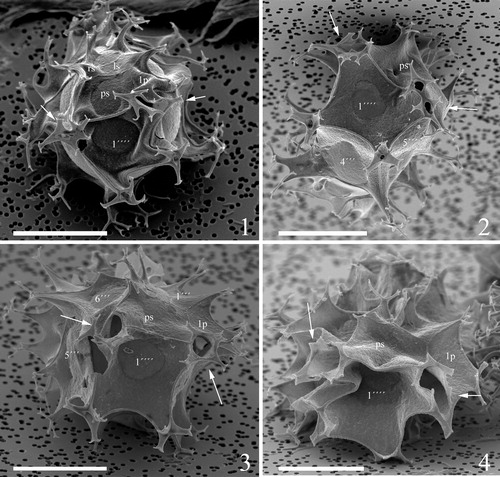
Plate 8. 1–4. Scanning electron micrographs of ventral views of four different specimens of Spiniferites elongatus from the Barents Sea with significant variation in the elevation of sutural crests, all showing the same displacement of one cingular width and configuration of sulcal plates. 1p = posterior intercalary plate, as = anterior sulcal plate, ls = left sulcal plate, ps = posterior sulcal plate, ras = right accessory plate, rs = right sulcal plate. Scale bars = 10 µm.
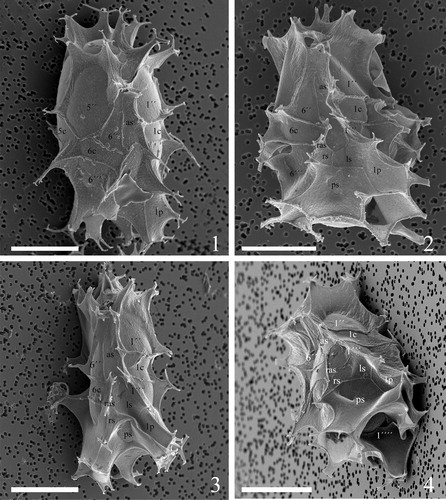
Plate 9. 1–4. Scanning electron micrographs of lateral and dorsal views of four different specimens of Spiniferites elongatus from the Barents Sea with significant variation in the elevation of sutural crests, showing the position of sulcal plate boundaries and a reduced archaeopyle corresponding to 3′′. Scale bars = 10 µm.
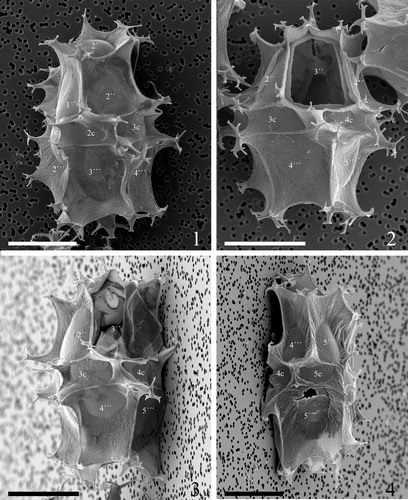
Plate 10. 1–4. Scanning electron micrographs of antapical views of four different specimens of Spiniferites elongatus from the Barents Sea with significant variation in the elevation of sutural crests, showing the antapical plate configuration with significant variation in fusion of ventral antapical multifurcate processes, indicated by white arrows on all specimens. 1p = posterior intercalary plate, ls = left sulcal plate, ps = posterior sulcal plate, rs = right sulcal plate. Scale bars = 10 µm.