Figures & data
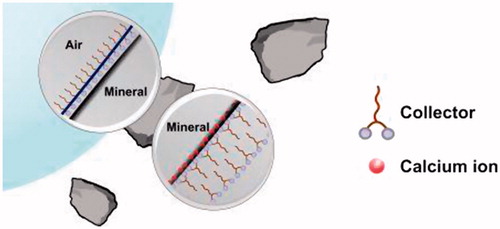
Figure 1. (Top-left) Sodium dodecanoate; (Top-right) Sodium N-dodecanoylglycinate; (Middle) Disodium N-dodecanoylaminomalonate; (Bottom-left) Disodium N-dodecanoylaspartate and (Bottom-right) Disodium N-dodecanoylglutamate.[Citation18]
![Figure 1. (Top-left) Sodium dodecanoate; (Top-right) Sodium N-dodecanoylglycinate; (Middle) Disodium N-dodecanoylaminomalonate; (Bottom-left) Disodium N-dodecanoylaspartate and (Bottom-right) Disodium N-dodecanoylglutamate.[Citation18]](/cms/asset/d4917ee1-4fd8-4d6c-996e-2e3e7cfc6b7e/ldis_a_1503547_f0001_b.jpg)
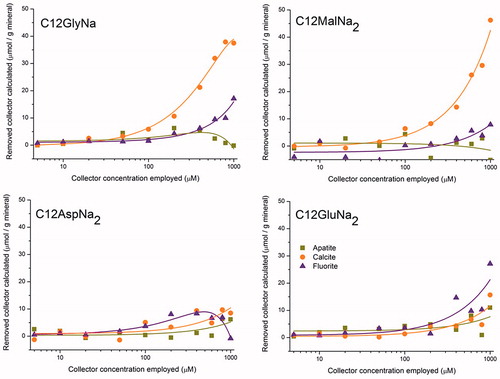
Figure 2. Flotation recovery for different minerals. apa, cal and flu stand for apatite, calcite and fluorite, respectively. The –c and –p terms indicate variation in concentration and pH, respectively.
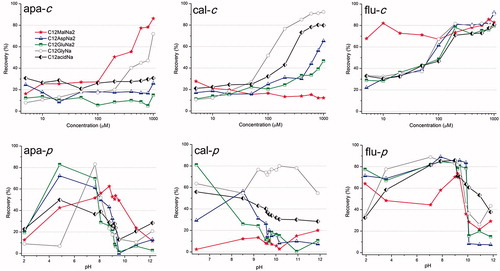
Figure 7. (a) Surfactant monolayer at the particle bubble interface. (b) A hypothetical ‘stable’ bilayer formation hindering efficient floatation. (c) A multilayer formation promoted by calcium ions (in red).
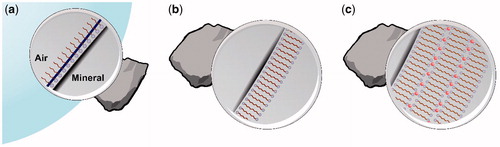
Table 1. Maximum distance between head groups in the double-headed collector molecules.
Table 2. Surface Ca-Ca shortest distance in calcium minerals.