Figures & data
Figure 2 FTIR spectra of the Cu trimer powder (a) and Cu trimer films before (b) and after (c) corrosion test.
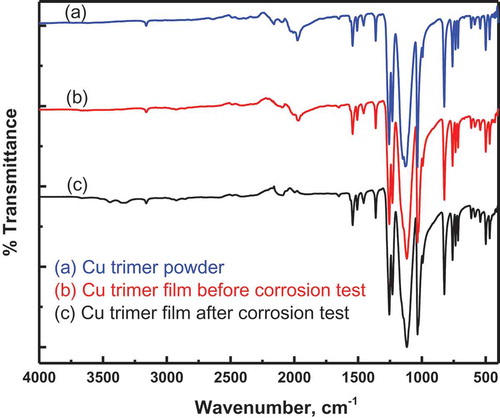
Figure 3 Top view of SEM images of uncoated aluminum alloy 3003 (a) vs that coated by the Cu trimer (b); EDX for the same coated substrate (c) used in B; side view of SEM images of two other aluminum alloy 3003s coated by the Cu trimer (d and e), showing both the substrate and coating simultaneously; and optical images of the luminescent Cu trimer film on the coated substrate before (f) and after (g) the corrosion test.
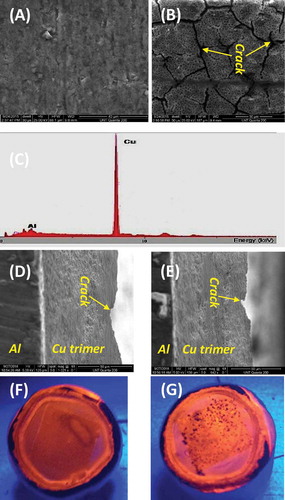
Figure 5 (a) Contact angle for uncoated aluminum surface; (b) contact angle for Cu trimer film on aluminum surface.
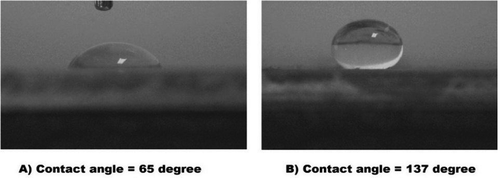
Figure 6 (a) Potentiodynamic polarization for uncoated (bare aluminum) and aluminum coated by a single coating of the Cu trimer and Ag trimer films; (b) potentiodynamic polarization for uncoated (bare aluminum) and aluminum coated by a double coating of the Cu trimer film.
Table 1 Potentiodynamic polarization plot data for uncoated aluminum and aluminum coated by Cu trimer
Figure 8 Tape test for adhesion measurements for films of the Cu trimer (left) vs Ag trimer (right) on aluminum substrates.

Table 2 Binding energy of the Al atom and distance between the Al atom and centroid of the M3 unit in a half-sandwich adduct of the Al atom with various unsubstituted and substituted cyclotrimers, according to M06/CEP-31G(d) simulations
Figure 9 Top: Geometries of the Al atom binding to the Cu (left) and Ag (right) trimers. Bottom: MEP surfaces of the trimers alone. Results are according to M06/CEP-31G(d) simulations. The Qzz quadrupole tensor values are labeled for the trimers alone, representing the highest magnitude (z axis is normal to the cylotrimer plane).
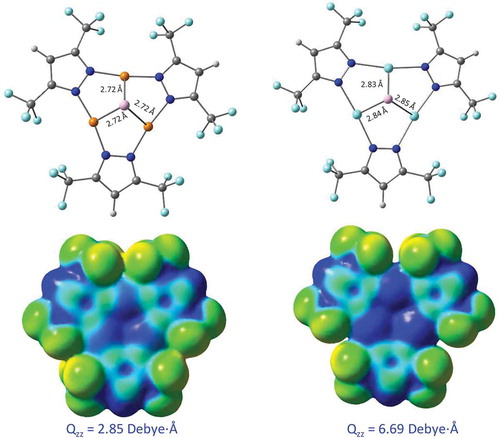
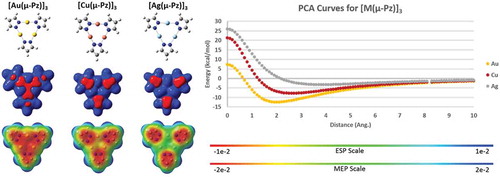
Figure 10 Left panel: Molecular structures (top), electrostatic potential showing positive and negative regions in space (ESP/middle), and molecular electrostatic potential (MEP/bottom) of unsubstituted coinage metal pyrazolate cyclotrimers. Right panel: Positive charge attraction (PCA) curves for the three trimers. Results are according to M06/CEP-31G(d) simulations using Gaussian 09 for ESP/MEP and using GAMESS for PCA. The Qzz quadrupole tensor values for the three trimers are –17, –15, and –20 Debye.Å with M = Cu, Ag, and Au, respectively, when unsubstituted, whereas the magnitude becomes positive upon CF3 substitution (see ).

![Figure 1 Molecular structure of {[3,5-(CF3)2Pz]Cu}3 (a.k.a., “Cu trimer”).](/cms/asset/23dce004-5ac0-450d-a644-1186ddc133ce/gcic_a_1559158_f0001_b.gif)