Figures & data
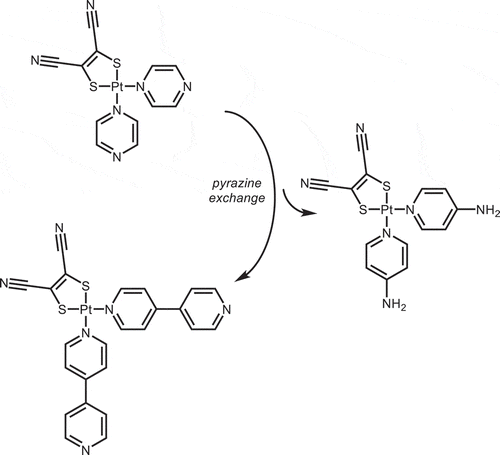
Table 1 Crystallographic data for compounds 1 and 3•C3H6O•2H2O
Scheme 1 (a) Syntheses of 1 (L = pyz) and 2 (L = 4,4ʹ-bpy) from [Pt(κ1-NN)4]2+ and (b) subsequent exchange of pyz ligands in 1 to form 2 or 3 (L = 4-ap)).
![Scheme 1 (a) Syntheses of 1 (L = pyz) and 2 (L = 4,4ʹ-bpy) from [Pt(κ1-NN)4]2+ and (b) subsequent exchange of pyz ligands in 1 to form 2 or 3 (L = 4-ap)).](/cms/asset/b90fb4ad-1bb5-429d-bacb-7535d05edf1f/gcic_a_1594205_sch0001_b.gif)
Figure 1 1H NMR spectra of Pt(pyz)2(mnt), 1 (a), Pt(4-ap)2(mnt), 3 (b) and Pt(4,4ʹ-bpy)2(mnt), 2 (c).
Table 2 Select bond lengths, angles, and torsion angles for Pt(pyz)2(mnt), 1, and Pt(4-ap)2(mnt), 3
Table 3 Select calculated bond lengths, angles and torsion angles for Pt(4,4ʹ-bpy)2(mnt), 2
Figure 2 Crystal structures (ellipsoids at 50%) of the H-bonded quadrangle containing two units of Pt(4-ap)2(mnt), 3, and two water molecules. Other solvent molecules omitted for clarity (top) and Pt(pyz)2(mnt), 1, (bottom).
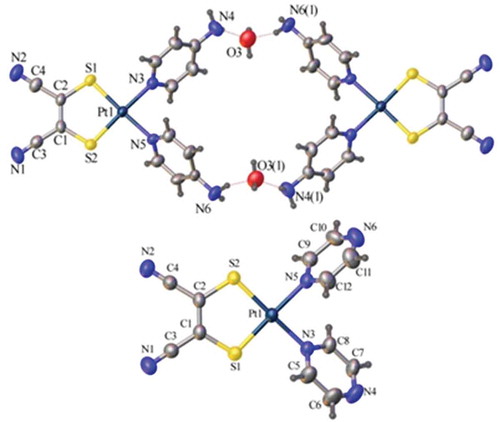
Figure 3 Crystal structures (ellipsoids at 50%) of 1 (top) and 3 (bottom) showing head-to-tail packing. H-atoms and solvent molecules omitted for clarity.
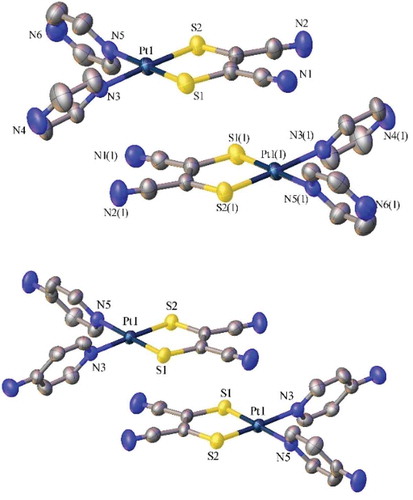
Table 4 Redox potentials for compounds 1–3 at a scan rate of 0.1 V/s. *Quasi-reversible while others are irreversible. All Ec and Ea values represent peak potentials.
Table 5 Comparison of experimental and calculated reduction/oxidation potentials for 1, 2, 3, and Pt(2,2ʹ-bpy)(mnt). Eabsexp was calculated according to Eabs = Eexp + 4.696 V to convert from a Ag/AgCl reference electrode to vacuum and Eabscalc = ΔGsol/nF where ΔGsol = ΔG°xsol – ΔGredsol. Experimental values for Pt(2,2ʹ-bpy)(mnt) taken from [50]
Figure 4 SOMO–1 (top) and SOMO (bottom) contours for 1 (left), 2 (middle), and 3 (right) (plotted with an isovalue of 0.02), supporting assignment of oxidation and reduction processes.
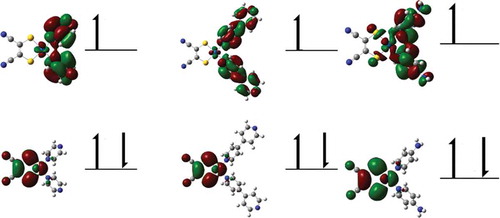
Figure 5 Cyclic voltammagram of 3 with five complete cycles from 0 to 1.5 V (red) and one cycle from 0 to 0.9 V (blue), indicating dependence of cathodic peak on oxidations at higher potential. Performed in acetonitrile using 0.1 M [n-Bu4N][PF6] electrolyte with a scan rate of 0.1 V/s.
![Figure 5 Cyclic voltammagram of 3 with five complete cycles from 0 to 1.5 V (red) and one cycle from 0 to 0.9 V (blue), indicating dependence of cathodic peak on oxidations at higher potential. Performed in acetonitrile using 0.1 M [n-Bu4N][PF6] electrolyte with a scan rate of 0.1 V/s.](/cms/asset/754bdfeb-c524-4e7f-9369-43511f2a07cb/gcic_a_1594205_f0005_oc.jpg)
Figure 6 Cyclic voltammagrams of 3 with increasing scan rate showing a negative shift in the oxidation potential dependent on scan rate.
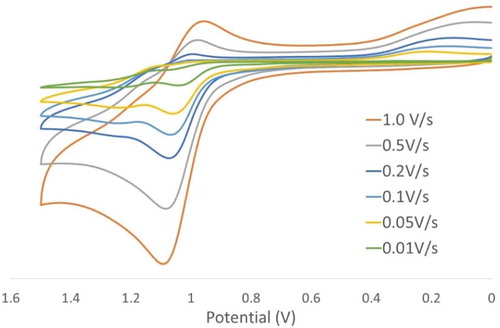
Figure 7 Cyclic voltammagram of 3 with increasing concentrations (0.2 mM, 0.4 mM, and 0.8 mM) indicating concentration dependence of cathodic peak at 0.32 V. Performed in acetonitrile using 0.1 M [n-Bu4N][PF6] electrolyte with a scan rate of 0.1 V/s.
![Figure 7 Cyclic voltammagram of 3 with increasing concentrations (0.2 mM, 0.4 mM, and 0.8 mM) indicating concentration dependence of cathodic peak at 0.32 V. Performed in acetonitrile using 0.1 M [n-Bu4N][PF6] electrolyte with a scan rate of 0.1 V/s.](/cms/asset/3f3cb1d7-e547-4fcf-92da-bdab52046c51/gcic_a_1594205_f0007_oc.jpg)
Figure 8 Electronic adsorption spectra of compounds 1–3 exhibiting diimine-based π-π* absorptions in the mid-UV region and tunable MMLL’CT in the near-UV extending into the visible region.
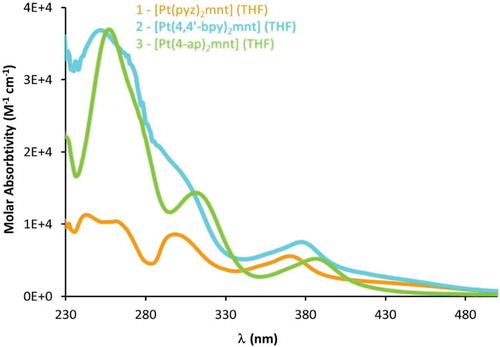
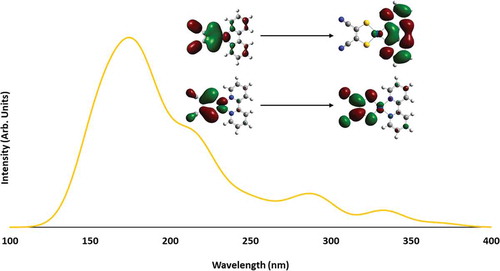
Figure 9 Calculated absorption spectra of 1 (a), 2 (b), and 3 (c) with orbital contours for the transition with the highest oscillator strength.
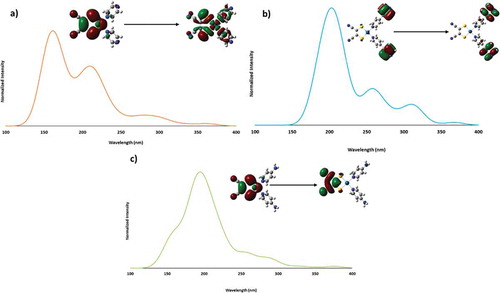
Table 6 Summary of lifetime data for frozen glassy solutions of 1 (10−3 M), 2 (10−4 M), and 3 (10−3 M) in acetone
Table 7 Summary of lifetime data for solid samples of 1, 2, and 3 at 77 K
Table 8 Comparison of calculated emission energies from the T1 and T2 states and excitation energy from S0 → T1
Figure 10 Photoluminescence excitation (left/thinner curves) and emission (right/thicker curves) spectra for frozen glassy solutions of 1, 2, and 3 in acetone.
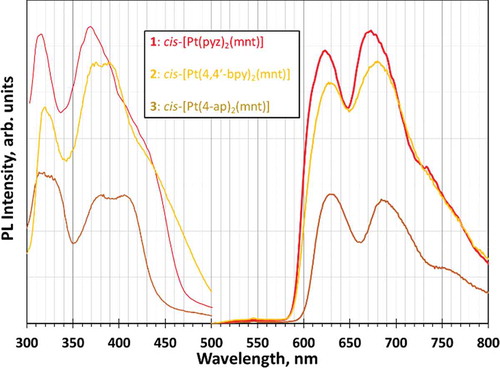
Figure 11 Photoluminescence excitation (left/thinner curves) and emission (right/thicker curves) spectra for solid samples of 1, 2, and 3 at 77 k.
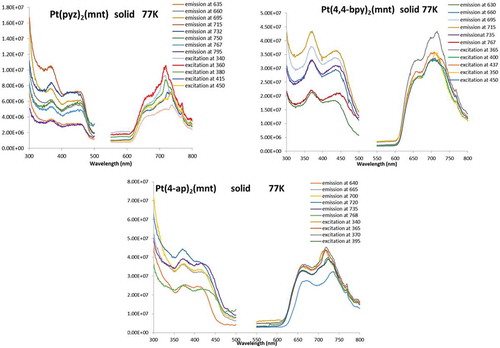
Table 9 Select bond lengths, angles, and torsion angles for Pt(2,2ʹ-bpy)(mnt). Experimental values for Pt(2,2ʹ-bpy)(mnt) taken from [47Citation47]
Figure 12 SOMO and SOMO-1 for the convergent Pt(2,2ʹ-bpy)(mnt) complex along with experimental oxidation and reduction values (in DMF) taken from [50] and calculated oxidation and reduction potentials.
![Figure 12 SOMO and SOMO-1 for the convergent Pt(2,2ʹ-bpy)(mnt) complex along with experimental oxidation and reduction values (in DMF) taken from [50] and calculated oxidation and reduction potentials.](/cms/asset/0f872000-cbb6-4a2d-b8c8-6100dba0ea3a/gcic_a_1594205_f0012_oc.jpg)
Figure 13 Calculated absorption spectra of Pt(2,2ʹ-bpy)(mnt) with orbital contours for the transition with the highest oscillator strength.