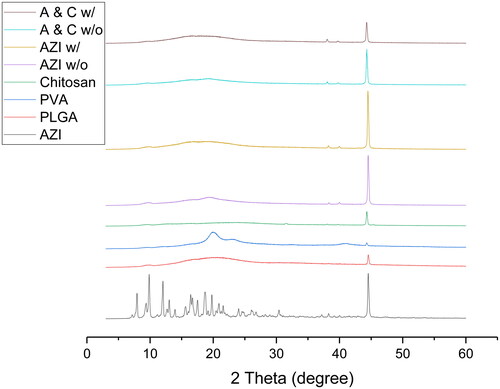
Figures & data
Table 1. Mean particle size, polydispersity index (PDI), zeta potential, encapsulation efficiency and drug loading of the two formulations (mean ± SD, n = 3).
Figure 1. Transmission electron micrographs of nanoparticles of the final two formulations – (a) azithromycin formulation (33,000× magnification), (b) azithromycin formulation (93,000× magnification), (c) azithromycin and chitosan formulation (33,000× magnification), (d) azithromycin and chitosan formulation (93,000× magnification).
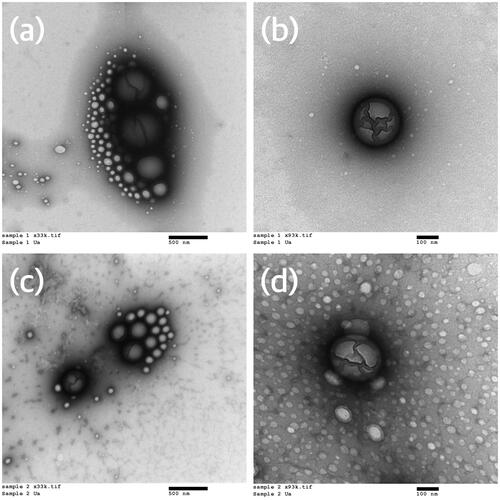
Figure 2. Scanning electron micrographs of spray-dried formulations – (a) azithromycin formulation without centrifugation (10,000× magnification), (b) azithromycin formulation without centrifugation (50,000× magnification), (c) azithromycin formulation with centrifugation (10,000× magnification), (d) azithromycin formulation with centrifugation (50,000× magnification), (e) azithromycin and chitosan formulation without centrifugation (10,000× magnification), (f) azithromycin and chitosan formulation without centrifugation (50,000× magnification), (g) azithromycin and chitosan formulation with centrifugation (10,000× magnification), (h) azithromycin and chitosan formulation with centrifugation (50,000 × magnification).

Table 2. X10, X50, X90, VMD and Span value of all four formulations (mean ± SD, n = 3).
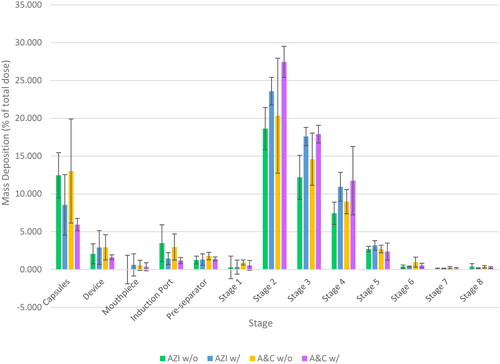
Table 3. Aerodynamic parameters of formulations delivered to the NGI® (mean ± SD, n = 3).