Figures & data
Figure 1. Cell growth in response to adenoviral infection. Cells numbers were counted in two sets of cells over a period of 3 days after plating. Each count is the average of three counts performed using a Coulter cell counter. In set 1, the viral construct added to the cells was left in the media over the duration of the experiment. In set 2, after 24 h of infection, the attached CrFK cells were washed with PBS to remove any free-floating virus and fresh media replaced. No major effect of adenoviral infection was noted on exponential cell growth at various MOIs (0, 5, 10, 20, 30 and 40).
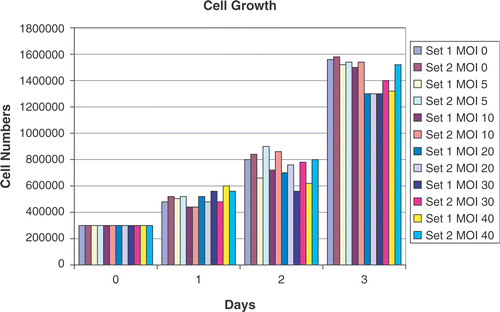
Figure 2. Transfection efficiency of adenoviruses. CrFK cells were infected with the Ad CMV fIL-12 + eGFP construct and green fluorescence detected flow cytometrically over 3 days, starting 24 h after addition of virus (day 1). A high transfection efficiency (90–95%) was noted at MOIs > 20. The high percentage of infected cells was maintained over the 3 days in set 1 (a). There was a slight decrease in percentage infection on day 3 in cells where the free-floating virus had been washed off (b).
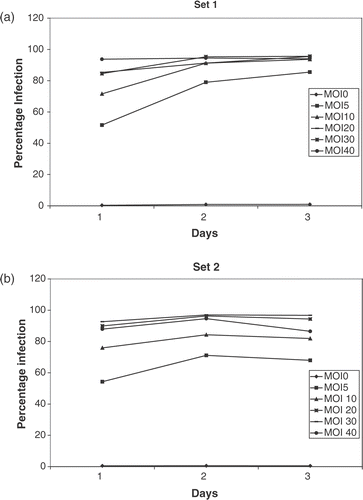
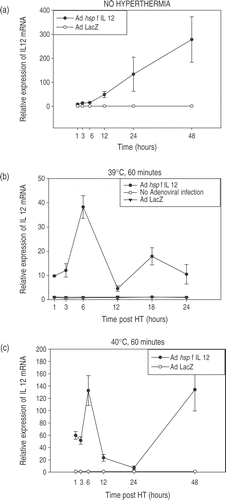
Figure 3. Temperature-dependent IL-12 expression. Profiles of temperature-dependent changes in IL-12 mRNA expression at specified time-points after hyperthermia. Cells were infected using the Ad hsp fIL-12 construct at MOI = 20. (a) Shows the minimal levels of expression at the early time points in unheated cells (kept in an incubator at 37°C, 5% CO2). (b−e) Show the profiles for heating at 39°C, 40°C, 41°C and 42°C for a duration of 60 min. Each temperature experiment was performed 2–3 times each in triplicate. The maximal expression of mRNA is seen at 6 h post-HT in all cases with a rapid decrease and then a secondary increase at 48 h. There is an ∼3.5-fold increase in maximal expression between 39°C, 40°C and 41°C. However, a supra-linear increase is seen at 42°C (note: log scale on y-axis). (f) Shows the maximal relative expression of IL-12 at the 6-h post-HT time point relative to temperature.
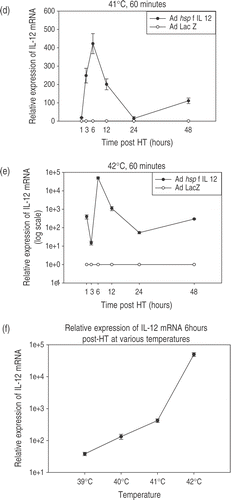
Table I. CrFK were infected at a MOI = 20 with the Ad hsp fIL-12 gene construct and heated at various non-cytotoxic temperatures for 60 min. The IL-12 mRNA relative expression relative to non-infected CrFK cells is presented as average ± SD. Each temperature experiment was performed 2–3 times each in triplicate.
Figure 4. Assessment of cell viability at higher temperatures. The trypan blue dye-exclusion test was used to assess cellular viability after being heated at 43°C and 44°C for 60 min. Direct hyperthermia induced cytotoxicity reduced the percentage of viable cells to ∼88% for 43°C and ∼73% for 44°C as early as 1 h post-HT and this percentage remained constant up to 24 h post-HT.
Figure 5. Effect of multiplicity if infection (MOI). CrFK cells were infected at various MOIs and heated at 40°C for 60 min. A dose-response relationship is seen (a) with the curve for maximum relative expression tending to flatten out at higher MOIs (b).
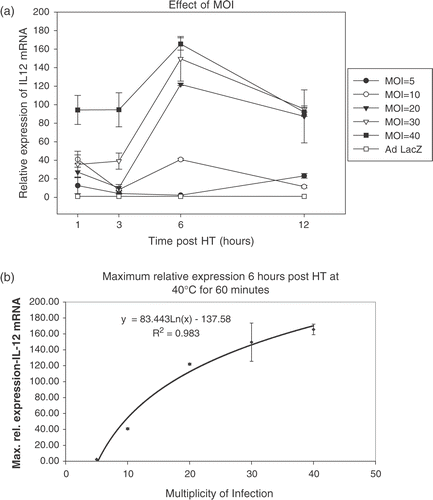
Figure 6. Induction of IFN-γ. Functionality of IL-12 was assessed by adding 100 μl of supernatants obtained from Ad hsp fIL-12 infected CrFK cells to Con A stimulated naïve feline PBMCs. These CrFK cells had been heated at 41°C for 60 min and supernatants were collected at 6, 12, 18 and 24 h post-HT. The PBMCs were lysed 12 h later (to allow action of IL-12 upon its target cells and the production of IFN-γ mRNA). The maximum induction was seen in the PBMCs in which supernatant from the 18 h post-HT CrFK cells had been added, suggesting that this sample contained the maximum amount of IL-12 protein.
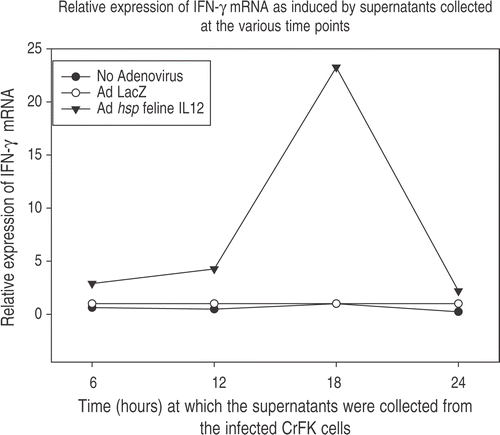
Table II. Supernatants from Ad hsp fIL-12 infected cells heated at 41°C for 60 min containing the IL-12 protein were collected at 6 and 12 h post-HT. These supernatants were added to naïve feline PBMCs in volumes of 100 or 200 μl to assess any dose response effect for IL-12 protein in inducing production of IFN-γ. At the earlier time point (6 h), when there would have been little IL-12 protein production, no difference was seen for the two doses, but by the 12 h time point an ∼2-fold increase in relative expression of IFN-γ mRNA was seen for the higher dose of added IL-12 protein.