Figures & data
Figure 1. Schematic of the experimental setup. HeLa cells were seeded at a concentration of 3.5 × 104 cells/well in the six wells of a 24-well plate shown in gray.
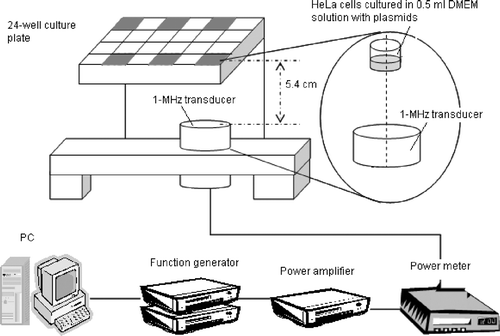
Table I. Parameters for experiments involving a fixed duty cycle of 50% with variable on- and off-times.
Table II. Parameters for experiments involving a fixed off-time (25 ms) with variable on-times.
Table III. Parameters for experiments involving a fixed on-time with variable off-times.
Figure 2. Effect of the PRF (fixed duty cycle with variable on-/off-times) on the percentage of viable cultured HeLa cells. Significant cell destruction compared with controls (p < 0.05, two-tailed t-test) is evident for PRFs between 2 and 2000 Hz. The temperature did not differ significantly among the groups for PRF equal to 2000 and 4000 Hz (#, p > 0.05, two-tailed t-test), but significantly difference in cell survival was found between them (*, p < 0.05, two-tailed t-test).
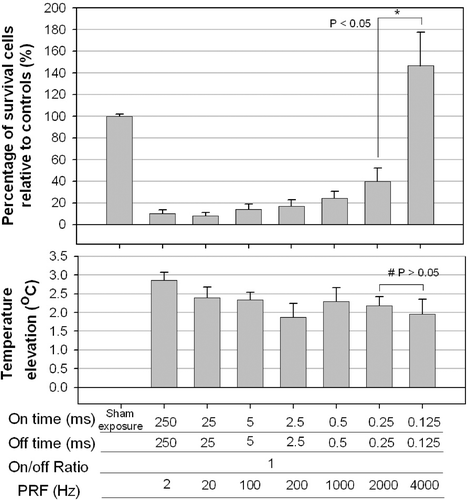
Figure 3. Effect of the on-time (and variable off-times) on the percentage of viable HeLa cells. Significant cell destruction compared with controls (p < 0.05, two-tailed t-test) is evident for on-times of 5, 25 and 250 ms. No significant cell destruction is evident for on-time of 0.5 and 2.5 ms. However, the temperature decreased with the on-time. Significant difference was found in cell destruction (*) between on-times of 5 and 2.5, but not in temperature elevation (#).
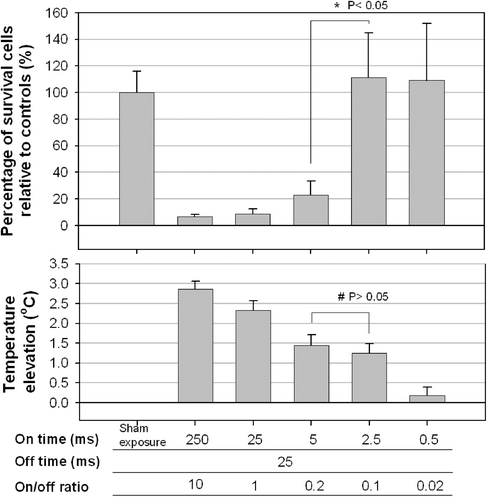
Figure 4. Effect of the off-times (pairs of a fixed on-time with an off-time that was either the same or 10 times longer) on the percentage of viable HeLa cells at 28 h after sonication. Significant cell destruction but with a temperature increase of less than 1.5°C compared with controls (p < 0.05, two-tailed t-test) is evident for on/-off-times of 25/250 and 250/2500 ms/ms were found.
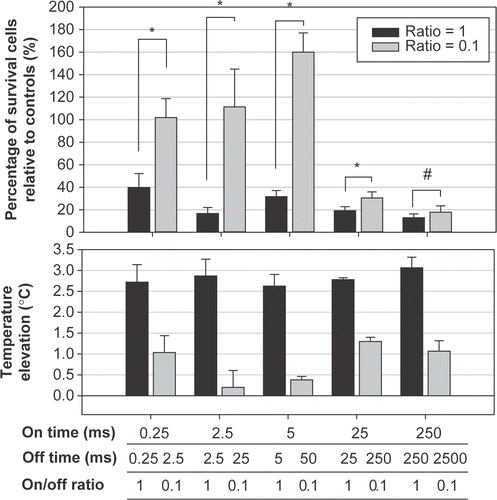
Figure 5. Effect of the fixed on-times but varied off-times on the survival ratio of different types of cultured cells at 4 h after sonication. * The survival ratio is significantly different from controls (p < 0.05, two-tailed t-test). Control: cells without ultrasound exposure. Each column represents the average of three trials of the combined result from nine samples per trial.
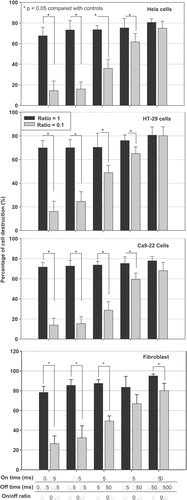
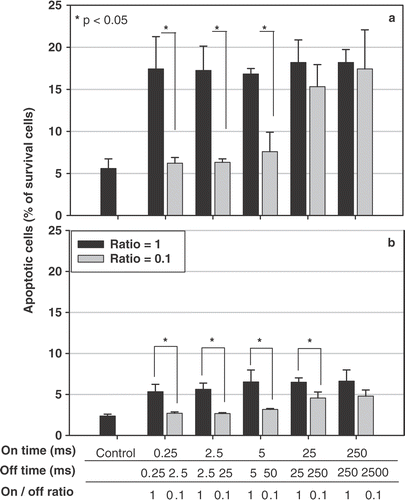
Figure 6. Ultrasound induces apoptotic cell death as a function of time, determined by flow cytometer. Hela cell line was sonicated using five paired parameters (on-off ratios of 1 and 0.1, with different on- and off-times). Four (a) and 28 (b) hours after the ultrasound exposure, the relative percentage of cell apoptosis, including both early apoptosis and secondary necrotic cells, were determined using the FITC-labeled annexin V kit. * : p < 0.05 (n = 3).