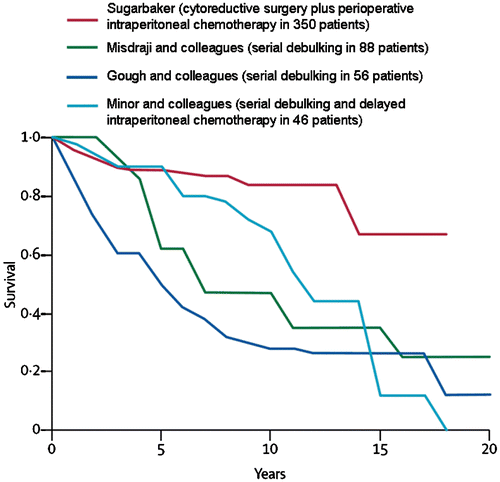
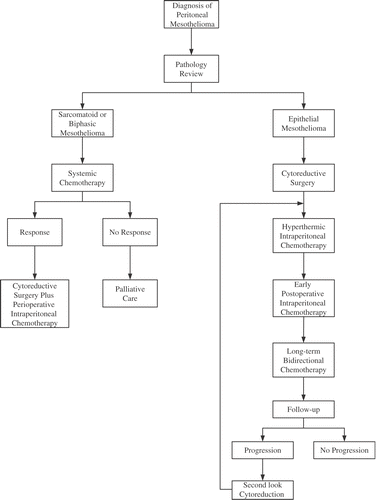
Figures & data
Figure 1. Small bowel sparing in a patient with mucinous adenocarcinoma arising in the appendix. Large volumes of cancer have accumulated in the omentum (omental cake), in the crevice between the undersurface of the right hemidiaphragm and liver, and by gravity within the pelvis. Cytoreductive surgical procedures have evolved to the extent that all visible evidence of disease can be surgically removed.
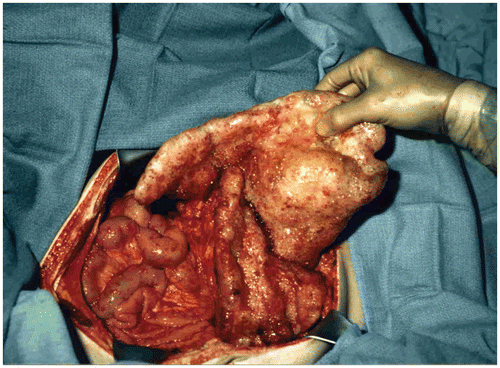
Figure 2. Pharmacokinetics of heated intraoperative intraperitoneal melphalan 120 mg in 3 litres of 1.5% dextrose peritoneal dialysis solution. Melphalan concentrations in peritoneal fluid, tumor nodules and plasma were determined by high-pressure liquid chromatography. The AUC ratio of peritoneal fluid to plasma was 25. The ratio of tumor nodules to plasma was 12.
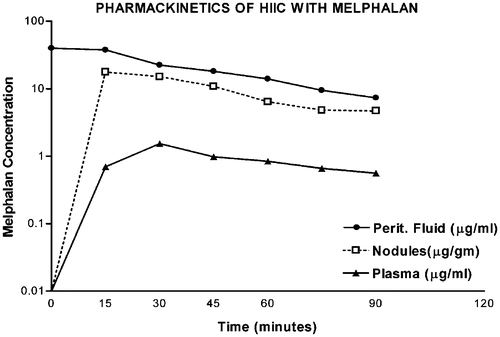
Table I. Chemotherapy agents used for HIIC.
Figure 3. Line diagram of the covered technique in HIIC. Using a heavy gauge monofilament suture the skin edges are elevated on a self-retaining retractor. A small cruciate incision is made in the clear plastic to allow the surgeon's double-gloved hand to reach all portions of the peritoneal cavity and optimize the distribution of the heated chemotherapy solution. Temperature probes are placed over the skin edge. An infusion catheter and three drainage catheters are used. The inflow catheter is usually placed beneath the right hemidiaphragm and above the right lobe of the liver so that the hottest solution does not directly flow onto the small bowel. One and often two smoke evacuators are placed beneath the clear plastic sheet in order to remove any possible chemotherapy aerosols and trap them in a charcoal filtration system.
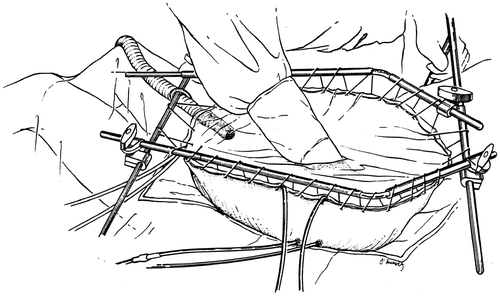
Figure 4. Temperature profile for HIIC. The line with open circles indicates the temperature maintained along the inflow catheter. The crossed lines show the temperature within the peritoneal cavity at the tip of the inflow catheter. The lines with stars show the temperature at a remote site, usually the base of the pelvis. The lines with diamonds show the core temperature usually within the oesophagus.
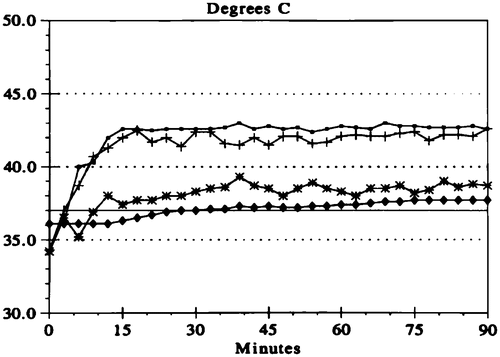
Figure 5. Pharmacokinetic study of intraperitoneal mitomycin C at 10 mg/m2 in 3 litres of 1.5% peritoneal dialysis solution. Mitomycin C levels were determined by high-pressure liquid chromatography in plasma and peritoneal fluid in a single patient. The data are representative of multiple similar studies performed in the operating room.
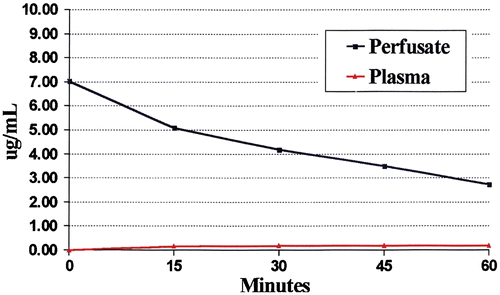
Figure 6. Pharmacodynamic study of intraperitoneal doxorubicin in a single patient. Fifteen milligrams per m2 of doxorubicin was used in 3 litres of 1.5% Dianeal. Doxorubicin levels were determined by high-pressure liquid chromatography in the peritoneal fluid, tumor nodules, and plasma at 15-min intervals for 90 min. The area under the curve ratio of peritoneal fluid to plasma doxorubicin was 110. The data are representative of multiple similar studies performed in the operating room.
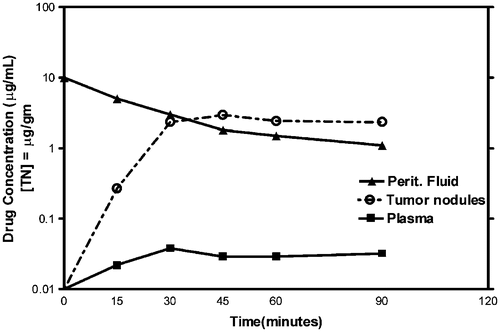
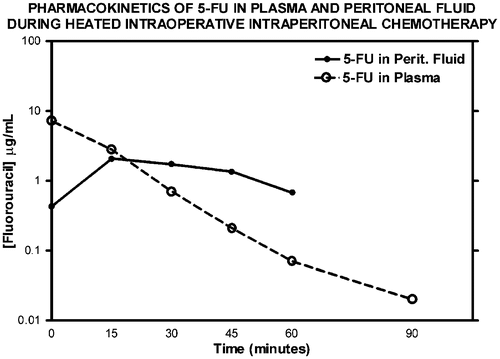
Figure 7. Pharmacologic study of systemic heated intraoperative 5-fluorouracil chemotherapy in a single patient. Systemic 5-fluorouracil at 400 mg/m2 was given simultaneously with intraperitoneal oxaliplatin at 140 mg/m2. The heat should increase the cytotoxicity of chemotherapy agents entering the tumour nodule from the plasma as well as entering by diffusion from the peritoneal fluid. The data are representative of multiple similar studies performed in the operating room.