Figures & data
Table I. Experimental groups for in vivo studies.
Figure 1. (a) murine fibrosarcoma cells (FSaII) and microvascular endothelial cells (MVEC) assessed by clonogenic cell survival, assay. *Indicates statistically significant difference from untreated control. **Indicates statistically significant difference from untreated control and heat alone. Cells were treated for 4 hours with 10–1000 ng/ml of soluble TNF or CYT-6091, or PT-cAu, before heating for 60 minutes at 42.5°C. Viability is expressed as mean ± SEM obtained from 5–6 different experiments and normalized to untreated control.
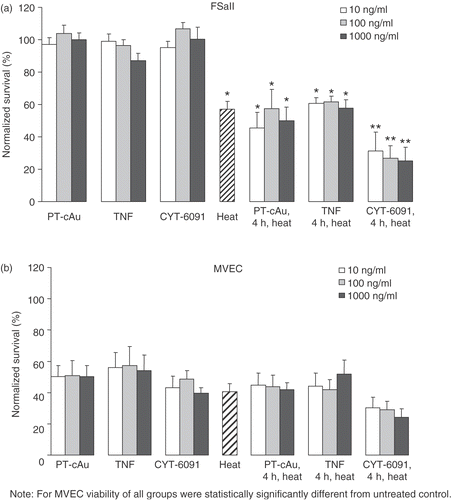
Figure 2. 86Rb uptake in murine fibrosarcoma tumors after intravenous administration of 250 µg/kg of soluble TNF or CYT-6091, or PT-cAu. Results are expressed as mean ± SEM of 4–5 animals and normalized to untreated controls. *Indicates statistically significant difference from untreated control.
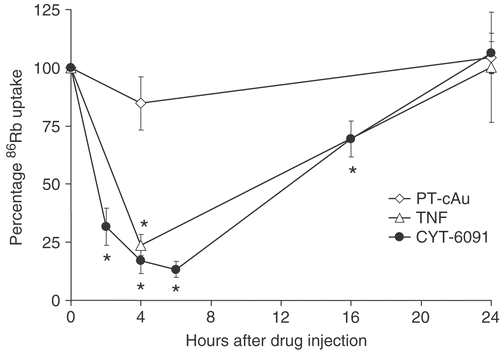
Figure 3. (a) Representative images of murine fibrosarcoma bearing hindlimb of C3H mice and the corresponding tumor perfusion defects imaged using contrast enhanced ultrasonography (bottom), 7 days post treatments. (b) Perfused murine fibrosarcoma tumor area obtained by quantification of contrast enhanced ultrasonographs, imaged 3 and 7 days post treatments. Results are expressed as mean ± SEM of 3 animals. *Indicates statistically significant difference from untreated controls.
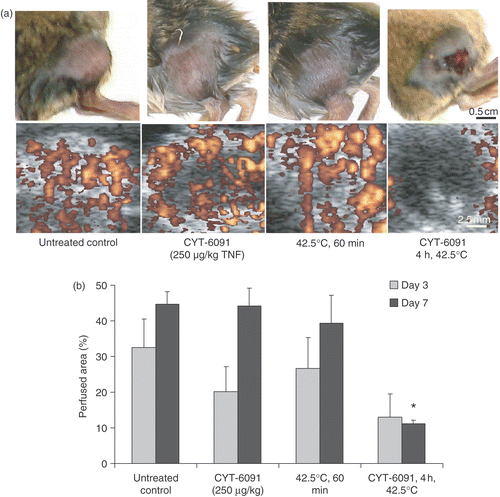
Figure 4. Murine fibrosarcoma tumor volume following hyperthermia and/or CYT-6091 treatments. Results are expressed as mean ± SEM of 5–6 animals and normalized to pretreatment volumes.
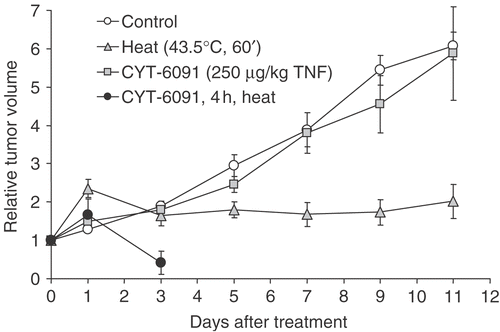
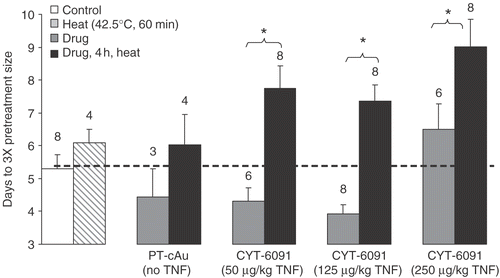
Figure 5. Days (mean ± SEM) for murine fibrosarcoma tumors to grow 3-fold their pretreatment volumes following various treatments shown. The numbers on the plot represent number of animals (N) in each group. In addition to the statistics shown, the results of the combination groups were found to be statistically significantly different from untreated control.