Figures & data
Figure 1. Tumor immunity depends on the generation of tumor-reactive T cells in the lymph node compartment and subsequent trafficking to the tumor microenvironment. Antigens derived from microbial pathogens or tumor cells in peripheral tissues are carried by dendritic cells to draining lymph nodes via the afferent lymphatic network. Naïve T cells enter lymph nodes across high endothelial venules (HEVs) and proliferate in response to antigen presentation by dendritic cells. Following exit of effector T cells via the efferent lymphatics, these cells circulate throughout the body in search of cognate target cells. The inset illustrates how fever-range thermal stress improves immune surveillance by increasing naïve T cell trafficking across HEVs. Thermal stress modifies the intravascular landscape in HEVs by increasing the density of trafficking molecules (CCL21 and ICAM-1) that support lymphocyte extravasation. Vessels within tumor sites are hypothesized to be non-permissive to effector T lymphocyte trafficking. Thus, effector T cells are physically segregated from tumor cell targets, providing one potential tumor escape mechanism. The role of thermal stress in regulating endothelial adhesion in tumor vessels remains to be determined.
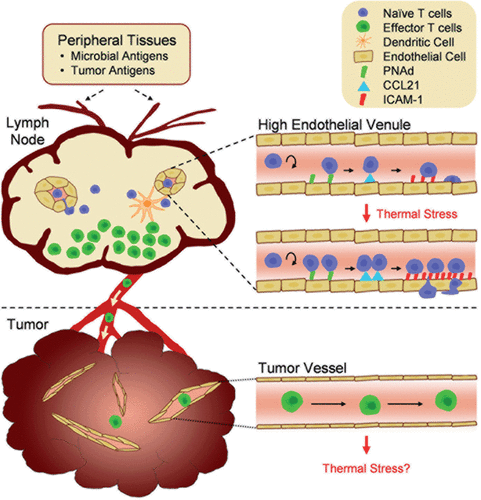
Figure 2. Fever-range thermal stress increases lymphocyte recruitment across HEVs. Photomicrographs show typical images of TRITC-labeled lymphocytes (red) associated with PNAd-positive HEVs (green) or infiltrated into the stroma of PLN tissue. NT, normothermic control; WBH, whole body hyperthermia. Bar = 50 µm.
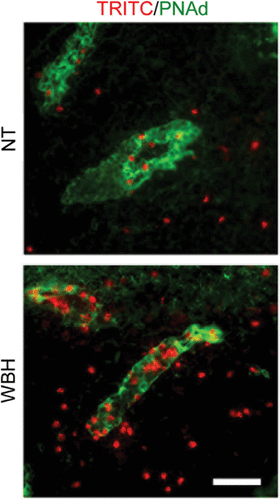
Figure 3. Fever-range thermal stress increases ICAM-1 display on HEVs of peripheral lymph nodes. Intravascular staining of ICAM-1 (red) and PNAd counterstaining (green). Bar = 50 µm.
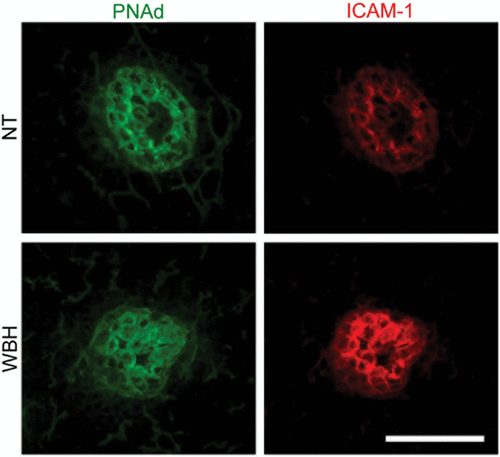
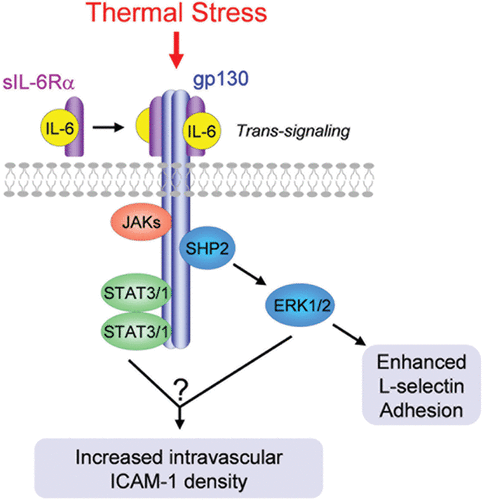
Figure 4. Fever-range thermal stress regulates lymphocyte and HEV adhesion through a common IL-6 trans-signaling mechanism. IL-6 trans-signaling is mediated by ligation of the membrane-anchored gp130 signal transducing subunit by a heterodimeric complex comprised of IL-6 and a soluble form of the IL-6 receptor α (sIL-6Rα) binding subunit. Downstream activation of ERK1/2 mediates thermal enhancement of L-selectin binding activity in lymphocytes. The signal transduction pathways underlying thermal induction of ICAM-1 density in HEVs are currently unknown.