Figures & data
Figure 1. Measured SAR patterns 5 mm deep inside liquid muscle phantom under 3 cm square DCC antenna driven with a clinically relevant power of 30 W at 915 MHz, with 0, 0.5, 1, and 2 mm diameter plastic catheters across one corner of the radiating slot.
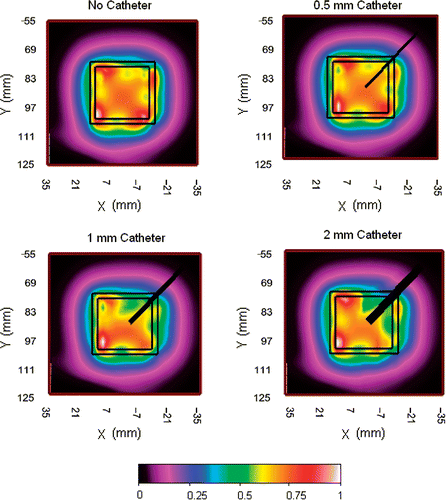
Figure 2. A view of the prototype TMS 4 × 4 sensor array with fiber-optic cabling for the 250 µm diameter IPITEK fiber-optic sensors to the readout electronics module.
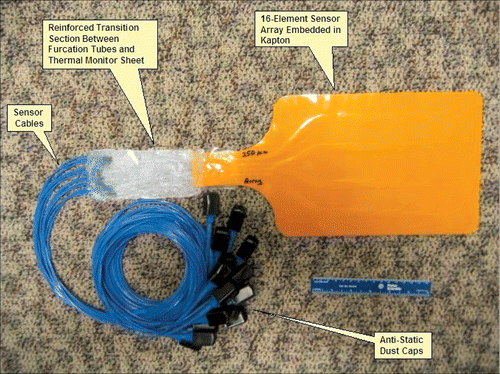
Figure 3. Conformity test showing (A) TMS position on torso phantom with (B) overlying water bolus, (C) air bladder vest and elastic overgarment as in typical CMA clinical setup; (D)–(E) Orthogonal views of the CT scans demonstrate excellent conformity and thermal contact to realistic curving and twisting body contour.
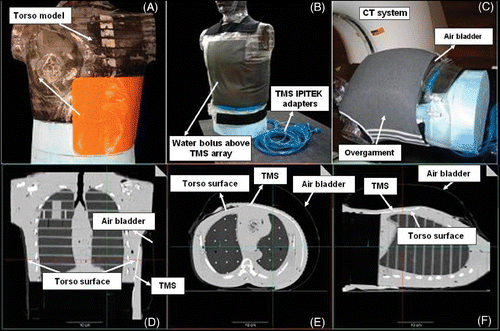
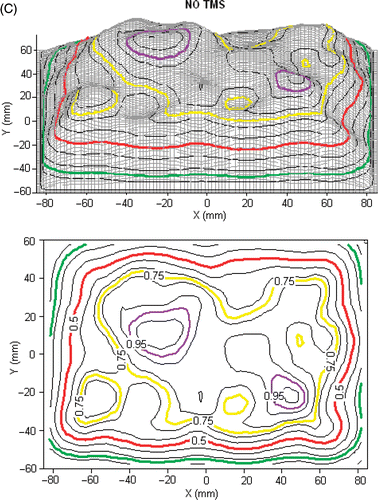
Figure 4. (A) Schematic diagram of the experimental setup for measuring SAR pattern at 5 mm depth inside muscle tissue equivalent liquid phantom irradiated with 915 MHz from a 6-element sub array of a CMA applicator; (B) SAR pattern with intervening 0.275mm thick TMS-250 sheet, superimposed above the outline of the 6 DCC antennas; (C) SAR pattern without TMS sheet.
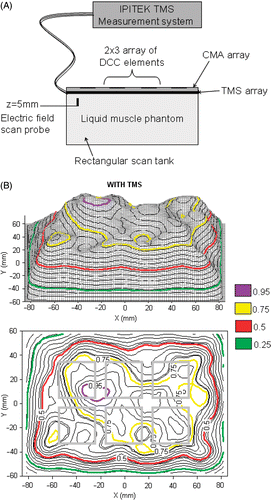
Figure 5. TMS probe self-heating from irradiation by Sonotherm 3.4 MHz ultrasound array hyperthermia applicator. (A) Experimental setup. (B) Comparison of standard thermocouple (TC) probes with TMS sensors reading tissue phantom temperature before and after application of maximal clinical power level. Note close correspondence of all probe temperatures at the interface after contact with the water bolus at 30 sec, and consistent 1–2°C self-heating of the Kapton sheet fiber-optic sensors during application of a high clinical power level of ultrasound at time 10–20 min.
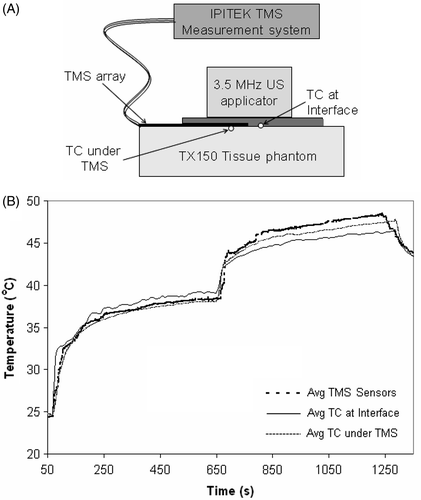
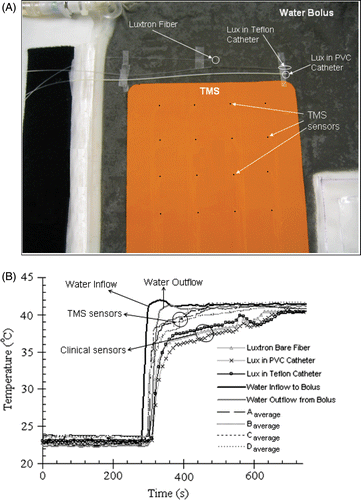
Figure 6. (A) Experimental set-up showing the placement of the TMS array and standard clinical temperature probes close together on a large equi-temperature water bolus sheet with rapidly circulating water (4 × 4 array of black dots indicate sensor location). (B) Comparison of the thermal response of TMS sensor array with standard clinical probes for a rapid change in circulating water temperature.