Figures & data
Figure 1. Finite element model meshes. This is an illustrative representation of both the (A) 2D finite element model used for simulating a 3 cm single internally cooled electrode in a 4 cm tumor (as denoted by the purple), and (B) the 3D finite element model used to simulate a 2.5 cm cluster electrode in a 4 cm tumor.
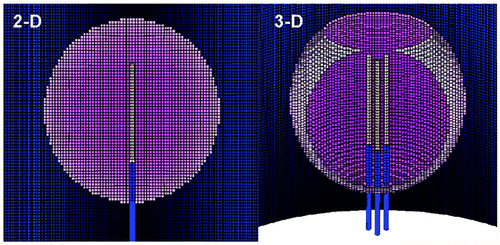
Figure 2. Effect of varying outer tissue thermal conductivity on RF heating for a 3 cm single electrode. This figure demonstrates the differences in the time required to achieve 50°C, using an internally cooled 3 cm single electrode, of the entire 3 cm tumor (0.5 W/m-°C) (without (top) and with (bottom) a 5 mm ablative margin) surrounded by fat (left, 0.23 W/m-°C), soft tissue (middle, 0.5 W/m-°C), and fluid (right, 0.7 W/m-°C), for varying inner tumor (y-axis) and outer tissue (x-axis) perfusions. Increasing the outer tissue thermal conductivity (fluid > soft tissue > fat) increases the time required to achieve ablation of the tumor alone from 6-8 min (fat) to up to 20 min (fluid), and at high perfusion states, complete ablation cannot be achieved. As an example, in treating renal cell carcinoma, with representative inner/outer perfusion characteristics (3 kg/m3-s and 3 kg/m3-s, respectively) denoted by the ‘R’, the outer thermal conductivity clearly influences the time required to achieve ablation.
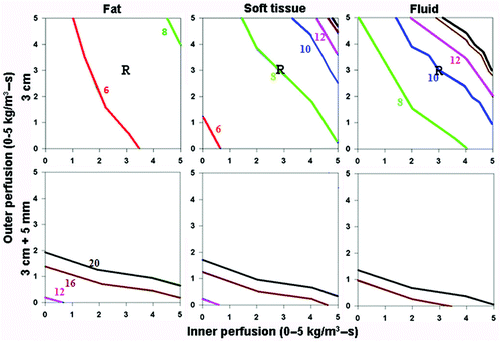
Figure 3. Effect of varying outer tissue thermal conductivity on RF heating for a 2.5 cm cluster electrode. This figure demonstrates the differences in the time required to achieve 50°C, using an internally cooled 2.5 cm cluster electrode, of the entire 4 cm tumor (0.5 W/m-°C) (without (top) and with (bottom) a 5 mm ablative margin) surrounded by fat (left, 0.23 W/m-°C), soft tissue (middle, 0.5 W/m-°C), and fluid (right, 0.7 W/m-°C), for varying inner tumor (y-axis) and outer tissue (x-axis) perfusions. Similar to findings in for a single electrode, perfusion demonstrates a dominant effect in all situations. Achieving an additional 5 mm ablative margin is also predominantly limited by high perfusions, though thermal conductivity does minimally effective ablation for surrounding fluid compared to fat/soft tissue. Inner perfusion has a dominant effect when trying to ablate the tumor alone, compared to including an ablative margin, where outer perfusion is more dominant. Increasing outer thermal tissue conductivity increases the time required for a given ablation, and can limit ablation success at higher perfusion states.
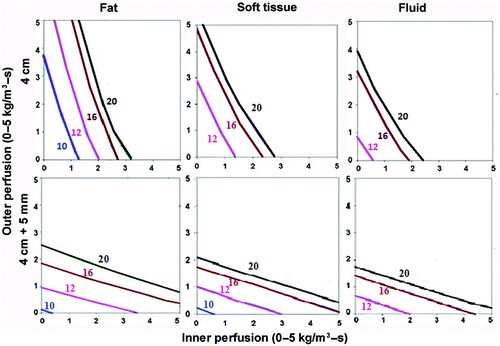
Figure 4. Effect of varying outer tissue electrical conductivity on RF heating for a 3 cm single electrode. This figure demonstrates the differences in the time required to achieve 50°C, using an internally cooled 3 cm single electrode, of the entire 3 cm tumor (0.5 S/m) (without (top) and with (bottom) a 5 mm ablative margin) surrounded by lung/bone (left, 0.1 S/m), soft tissue (middle, 0.5 S/m), and kidney (right, 3.3 S/m), for varying inner tumor (y-axis) and outer tissue (x-axis) perfusions. Increasing the outer tissue electrical conductivity (kidney > soft tissue > lung/bone) increases the time required to achieve ablation of the tumor alone from 6–8 min (lung/bone) to up to 20 min (kidney), and at certain high perfusion states, complete ablation cannot be achieved. Again, when trying to achieve an ablative margin, outer perfusion is more dominant, though in the absence of perfusion, outer thermal conductivity affects the time required to ablate the tumor.
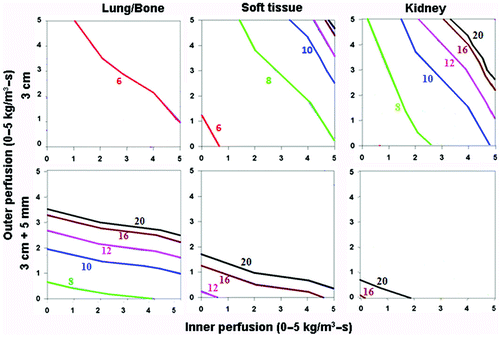
Figure 5. Effect of varying inner tissue electrical conductivity on RF heating for a 3 cm single electrode. This figure demonstrates the differences in the time required to achieve 50°C, using an internally cooled 3 cm single electrode, of both 3 (top) and 4 (bottom) cm tumors including a 5 mm ablative margin, infused with adjuvant saline (left, 4.0 S/m) or surrounded by soft tissue (right, 0.5 S/m), for varying inner tumor (y-axis) and outer tissue (x-axis) perfusions. Increasing the inner tissue electrical conductivity (adjuvant saline > soft tissue) decreases the time required to achieve ablation of the tumor alone from 12–20 min to up to 8 min, and increases the range of inner/outer perfusions at which tumors can be completely ablated. As an example, in hepatocellular carcinoma, with representative inner/outer perfusion characteristics (hypervascular HCC with an inner perfusion of 3.3 kg/m3-s, surrounded by cirrhotic liver with an outer perfusion of 1 kg/m3-s) denoted by the ‘H’, the outer thermal conductivity clearly influences the time required to achieve ablation, if this can be achieved at all.
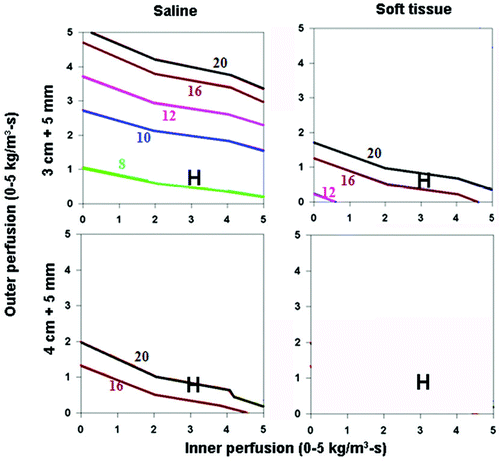
Figure 6. Comparing the effects of varying electrical and thermal conductivity for ‘best’ and ‘worst’ case scenarios for a 3 cm single electrode. This figure demonstrates the differences in the time required to achieve 50°C for varying inner tumor and outer tissue perfusions, using an internally cooled 3 cm single electrode for 3–5 cm tumors, with the ‘best’ (top, simulating adjuvant saline injecting in tumor (electrical inner and outer conductivity of 4.0 and 0.5 S/m, respectively), surrounded by fat (thermal inner and outer conductivity of 0.5 and 0.23 W/m-°C, respectively)) and ‘worst’ (bottom, simulating RCC in normal kidney (electrical inner and outer conductivity of 0.5 and 3.3 S/m, respectively), surrounded by ascites (thermal inner and outer conductivity of 0.5 and 0.7 W/m-°C, respectively)) scenarios based upon varying thermal and electrical conductivities. Significant differences can be seen between the ‘best’ and ‘worst’ combinations of electrical and thermal conductivity on the times required to achieve ablation and the range of tissue perfusions at which ablation can be achieved. For example, all 3 cm tumors regardless of perfusion can be ablated, compared to only those with lower inner/outer perfusion for 5 cm tumors. A similar pattern is seen when also trying to achieve an ablative margin.
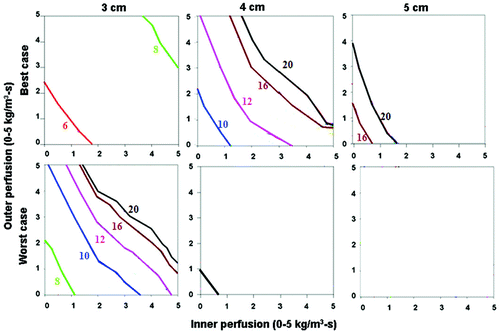
Figure 7. Comparing the effects of varying electrical and thermal conductivity for ‘best’ and ‘worst’ case scenarios for a 2.5 cm cluster electrode. This figure demonstrates the differences in the time required to achieve 50°C for varying inner tumor and outer tissue perfusions, using an internally cooled 2.5 cm cluster electrode for 4–5 cm tumors, with the ‘best’ (top, simulating adjuvant saline injecting in tumor (electrical inner and outer conductivity of 4.0 and 0.5 S/m, respectively), surrounded by fat (thermal inner and outer conductivity of 0.5 and 0.23 W/m-°C, respectively)) and ‘worst’ (bottom, simulating RCC in normal kidney (electrical inner and outer conductivity of 0.5 and 3.3 S/m, respectively), surrounded by ascites (thermal inner and outer conductivity of 0.5 and 0.7 W/m-°C, respectively)) scenarios based upon varying thermal and electrical conductivities. As for the single electrode, significant differences can be seen between the ‘best’ and ‘worst’ combinations of electrical and thermal conductivity on the times required to achieve ablation and the range of tissue perfusions at which ablation can be achieved.
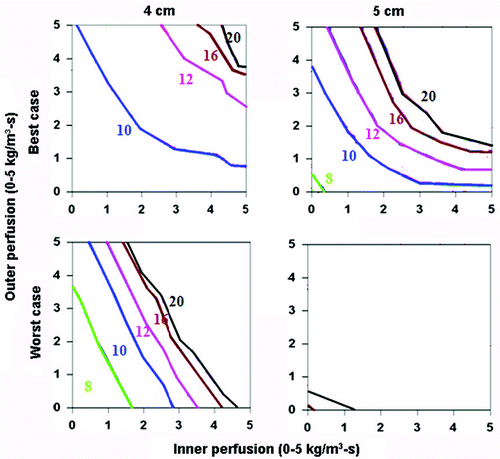
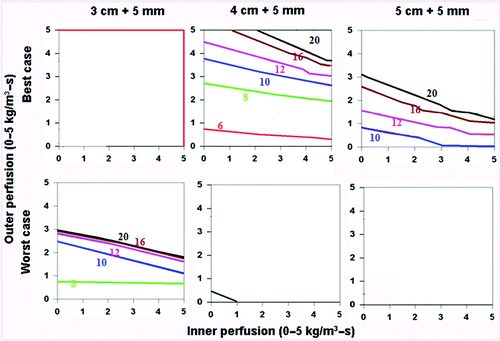
Figure 8. Comparing the effects of varying electrical and thermal conductivity for ‘best’ and ‘worst’ case scenarios. This figure demonstrates the differences in the time required to achieve 50°C for varying inner tumor and outer tissue perfusions, using an internally cooled 2.5 cm cluster electrode for 3–5 cm tumors with a 5 mm ablative margin, with the ‘best’ (top, simulating adjuvant saline injecting in tumor (electrical inner and outer conductivity of 4.0 and 0.5 S/m, respectively), surrounded by fat (thermal inner and outer conductivity of 0.5 and 0.23 W/m-°C, respectively)) and ‘worst’ (bottom, simulating RCC in normal kidney (electrical inner and outer conductivity of 0.5 and 3.3 S/m, respectively), surrounded by ascites (thermal inner and outer conductivity of 0.5 and 0.7 W/m-°C, respectively)) scenarios based upon varying thermal and electrical conductivities. Additionally, the ability to achieve ablation of a 5 mm margin is more dependent on outer perfusion (as demonstrated by the horizontally oriented isotherms) compared to achieving complete ablation without a margin (which is more dependent on inner perfusion, see for example).