Figures & data
Figure 1. Schematic representation of the experimental protocol described in the Materials and methods section. Group 1 was treated with gemcitabine (30 μM, 24 h), group 2 received heat treatment (43°C, 1 h) 24 h before treatment with gemcitabine, group 3 received heat treatment simultaneously with gemcitabine treatment, group 4 received heat treatment 24 h after the treatment with gemcitabine, and group 5 received heat treatment 48 h after the treatment with gemcitabine. After all treatments, cell viability was determined by a WST-8 assay, the numbers of cells were counted, and a qualitative analysis of cell death was performed by flow cytometry.
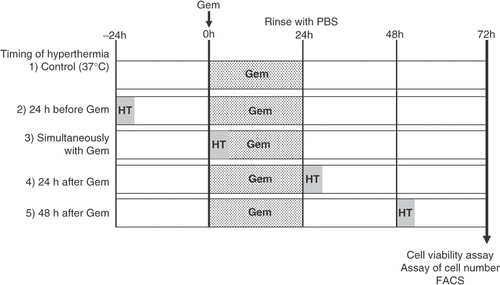
Figure 2. Cytotoxic effect of gemcitabine in pancreatic carcinoma cells. AsPC-1 and MIAPaCa-2 cells were treated with various concentrations (0–30 μM) of gemcitabine for 24 h. After treatment, cells were rinsed with phosphate-buffered saline and left for 48 h in the incubator at 37°C. Cell viability was measured by an MTT-based WST-8 assay. Values represent mean ± SEM of triplicate samples of a representative experiment; similar results were obtained in three independent experiments. *P < 0.05, compared with the control in AsPC-1 cells (a). **P < 0.0001, compared with the control in MIAPaCa-2 cells (b).
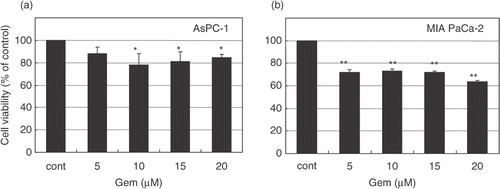
Figure 3. Heat treatment enhanced the cytotoxicity of gemcitabine. AsPC-1 and MIAPaCa-2 cells received heat treatment in combination with gemcitabine treatment (30 μM) at various relative times. Cell viability was measured by an MTT-based WST-8 assay. Values represent mean ± SEM of four samples of a representative experiment; similar results were obtained in three independent experiments. *P < 0.0001, compared with the control in AsPC-1 cells (a). **P < 0.005, compared with the control in MIAPaCa-2 cells (b).
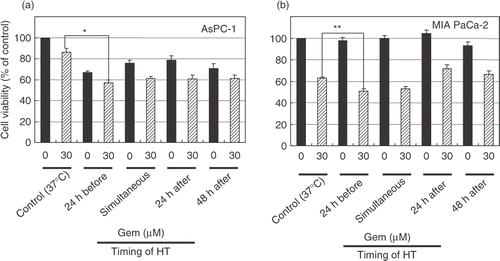
Figure 4. Apoptosis and necrosis induced by heat treatment with gemcitabine treatment. Heat treatment enhanced cell death in pancreatic carcinoma cells. The cell death pattern for AsPC-1 and MIAPaCa-2 cell lines was assessed by qualitative flow cytometry using Annexin-V and propidium iodide. The number in the lower left indicates living cells, upper left indicates propidium iodide-positive, lower right indicates Annexin V-positive, and upper right indicates both Annexin V- and propidium iodide-positive. (a) AsPC-1 cells were treated with heat 24 h before the treatment with gemcitabine at various relative times and the resulting apoptotic cell death patterns are shown. This experiment was performed twice with similar results. (b) In the case of MIAPaCa-2 cells, heat treatment performed 24 h before gemcitabine treatment also enhanced apoptosis. This experiment was performed twice with similar results.
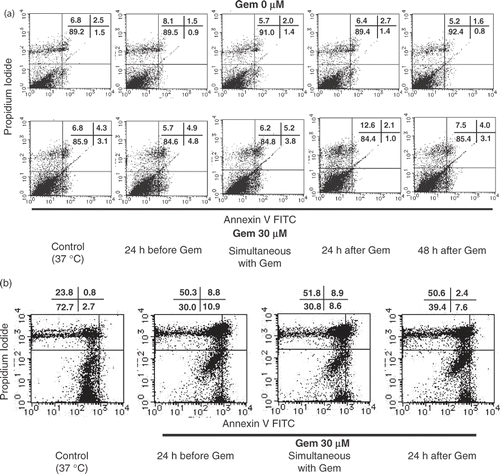
Figure 5. The effect of gemcitabine on NF-κB activation was studied by electrophoretic mobility shift assay. AsPC-1 and MIAPaCa-2 cells were exposed to 30 μM gemcitabine for various periods. At the indicated time, nuclear extracts were prepared and electrophoretic mobility shift assays for NF-κB binding activity were performed. Gemcitabine increased NF-κB binding activity in both cell lines. In AsPC-1 cells, gemcitabine increased NF-κB binding activity between 12 and 24 h. On the other hand, in MIAPaCa-2 cells, gemcitabine increased NF-κB binding activity as early as 3 h.
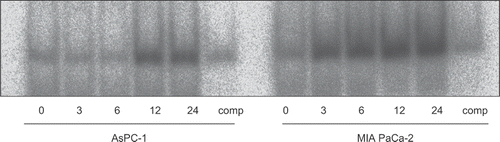
Figure 6. Gemcitabine-induced NF-κB was decreased by the combination therapy with heat treatment. The binding activity of NF-κB was assessed after 12 h of gemcitabine treatment or 24 h of heat treatment. AsPC-1 cells were exposed to four kinds of stimuli. The results for no treatment (control) (lane 1), treatment with gemcitabine alone (30 μM) (lane 2), treatment with heat alone (43°C, 1 h) (lane 3), and heat treatment combined with gemcitabine treatment (lane 4) are shown.
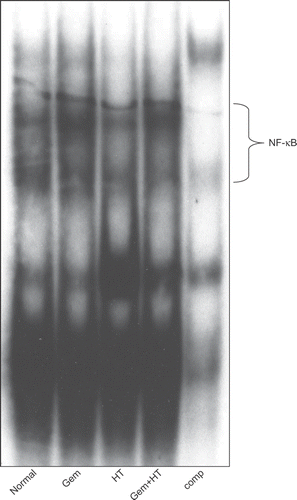
Figure 7. The status of NF-κB activity was determined by immunocytochemical staining using an antibody specific for p65. AsPC-1 cells were grown on gelatin-coated 8-well chambered glass slides and treated with heat 24 h before the treatment with gemcitabine (30 μM). The results for no treatment (control) (a), treatment with gemcitabine (30 μM) (b), treatment with heat (43°C, 1 h) (c), and heat treatment combined with gemcitabine treatment (d) are shown. In the untreated cells (control), the p65 subunit was localized exclusively in the cytoplasm. On the other hand, treatment with gemcitabine induced the translocation of p65 into the nucleus. Heat treatment inhibited the gemcitabine-induced translocation of NF-κB.
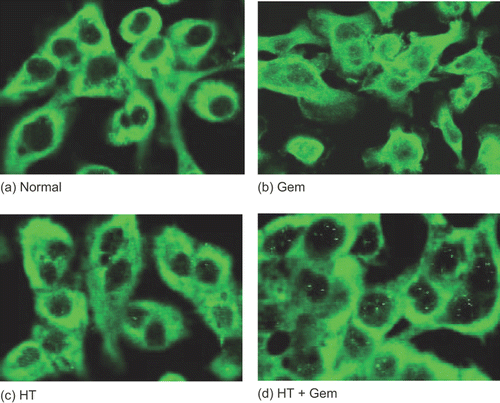
Figure 8. The effect of heat treatment in gemcitabine-mediated cytotoxicity. When heat treatment was given 24 h before the treatment with gemcitabine, a significant decrease in the total number of cells was seen compared with treatment with gemcitabine alone. Values represent mean ± SEM of three samples of a representative experiment; similar results were obtained in three independent experiments. *P < 0.05, compared with the control. **P < 0.01, compared with the control in AsPC-1 cells.
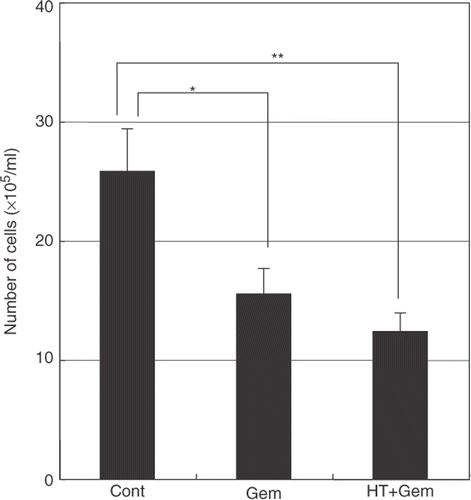
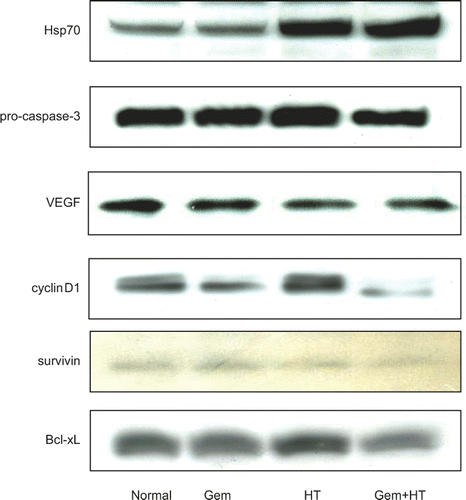
Figure 9. The expression levels of Hsp70, pro-caspase-3, VEGF, cyclin D1, and anti-apoptosis proteins. Cells were treated with heat 24 h before the treatment with gemcitabine (30 μM). Whole cell extracts were analyzed by western blotting. The results for no treatment (control) (lane 1), treatment with gemcitabine alone (30 μM) (lane 2), heat treatment alone (43°C, 1 h) (lane 3), and heat treatment combined with gemcitabine treatment (lane 4) are shown. Significant levels of Hsp70 were induced by heat treatment in MIAPaCa-2 cells. In contrast, gemcitabine treatment alone did not affect the protein levels of Hsp70. The levels of VEGF, pro-caspase-3, cyclin D1, and anti-apoptosis protein were decreased by heat treatment combined with gemcitabine treatment.