Figures & data
Figure 1. (a) The optimised structure of NGO at B3LYP/6-31 g*, containing epoxy, hydroxyl and carboxylic acid functional groups; (b) the position of AA on the NGO (Gly–NGO is showed as the representative example) and (c) The graphitic, pyridinic and pyrrolic nitrogen atoms.
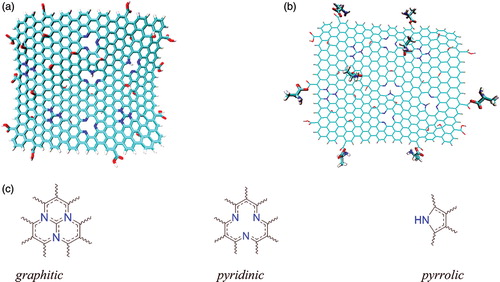
Figure 2. Binding energies between the TXL and AA-NGO systems obtained by docking studies. The binding energy related to the NGO is provided for the sake of comparison. The His and Trp grafted NGOs are showing the most affinity toward the TXL based on the calculated binding energies.
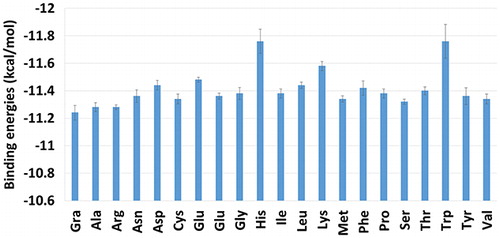
Figure 3. (a) Binding free energy between the TXL/(AA-)NGO systems calculated by MM/GBSA approach; (b) Binding energy differences between 42 and 37 °C (E42 – E37). Based on the results Trp is showing most affinity to the TXL at lower temperature in comparison with higher one. The Gly, Ile and Lys are acting in a similar way. They show more affinity to the TXL at lower temperature.
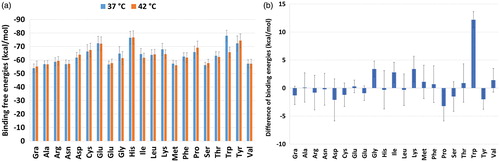
Figure 4. Sandwich-like AA–NGO/TXL/AA–NGO system. The AA–NGOs were positioned 15 Å away from each other (red = TXL, green = water molecules). (The colored image is available in online version.)
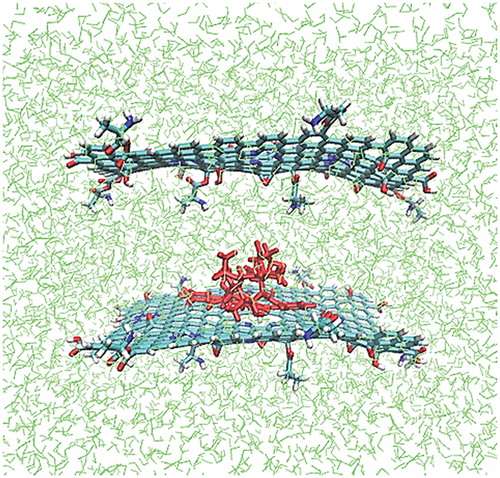
Figure 5. (a) Binding energies calculated for the sandwich-like systems at two studied temperatures (37 and 42 °C); (b) Binding energy differences = E42 – E37. The Asp–, Gly–, Thr– and Tyr–NGO are showing more affinity toward the TXL in lower temperature in comparison with hither temperature.
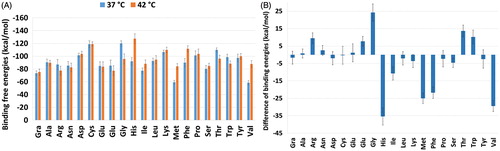
Figure 6. (a) Hydrogen bonding between the TXL and Gly–NGOs during MD simulation process. More hydrogen bonding interaction is lower temperature (I) are observable. (b) Representative hydrogen bonding interaction between the TXL and Gly–NGOs. Both OH moiety of the NGO and O of the Gly have contribution in the hydrogen bonding interaction with the TXL. (c) Distance between centre of mass of the two Gly–NGO sheets during MD simulation process. I and II are implying to 37 and 42 °C, respectively. At lower temperature the sheets have shorter distance. At higher temperature the sheets prefer to separate from each other.
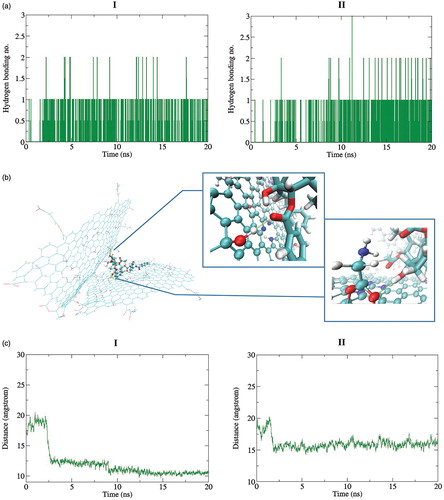
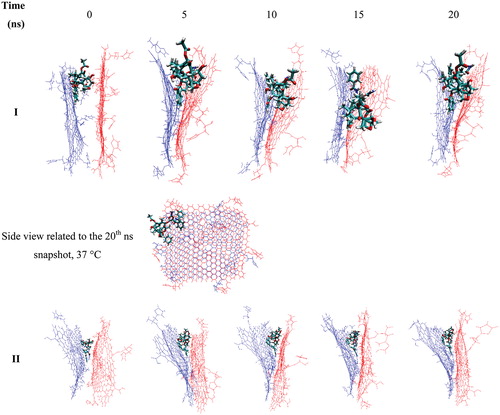
Figure 7. The change in the position of two Gly–NGO sheets during MD simulation process at 37 (I) and 42 °C (II), respectively. Snapshots for selected trajectories are presented. At higher temperature the sheets prefer to go away from each other that make it easy to release the TXL.
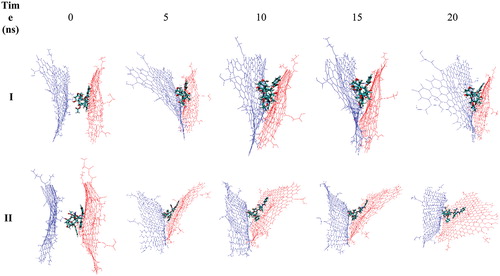
Figure 8. Snapshots related to selected trajectories of the sandwich-like Gly–NGO/TXL/Gly–NGO at 37 (I) and 42 °C (II) temperatures. At lower temperature (I) two Gly–NGO sheets are close to each other which is forcing the TXL to leave the system. At higher temperature (II) two mentioned sheets have enough distance from each other to keep the TXL inside the system and prevent it from leaving the system.