Figures & data
Table 1. Exemplar guidelines for the maximum injection volumes of experimental compounds in mice, rats, rabbits and humans (of the given FDA reference body weights) on the basis of the administration route, as used by the Duke University Medical Center in Durham, NC, USA [Citation47].
Figure 1. Equilibrium temperature profile ΔT(r) within and surrounding a sphere of radius R of a medium of thermal conductivity within a medium of thermal conductivity
, after prolonged exposure to an energy source depositing power P into the sphere [Citation74]. The solid lines represent solutions to EquationEquation (8)
(8a) for values of the ratio
/
ranging from 1.0 to 2.0, plotted in the reduced coordinate system where r′ = r/R, and ΔT′(r′) = 3
ΔT(r)/PR2.
![Figure 1. Equilibrium temperature profile ΔT(r) within and surrounding a sphere of radius R of a medium of thermal conductivity within a medium of thermal conductivity , after prolonged exposure to an energy source depositing power P into the sphere [Citation74]. The solid lines represent solutions to EquationEquation (8)(8a) for values of the ratio / ranging from 1.0 to 2.0, plotted in the reduced coordinate system where r′ = r/R, and ΔT′(r′) = 3 ΔT(r)/PR2.](/cms/asset/a4012b60-ceaf-4e8a-a135-68289a4a49f9/ihyt_a_1365953_f0001_c.jpg)
Figure 2. Illustrative bioheat model calculations in the steady-state, zero perfusion, zero metabolic heat generation limit, for a 60 kg human, as per EquationEquation (10)(9) , showing ΔTR isotherms in the Vi-c plane, where ΔTR is the equilibrium temperature at the surface of a sphere of radius R containing a uniform distribution of heat-evolving magnetic nanoparticles. Assumed parameters are: intrinsic loss power ILP = 3.0 nHm2/kgFe; dispersion factor v = Vd/Vi = 2.4; thermal conductivity of the surrounding medium λ2 = 0.52 W/Km; and activation field amplitude H = 5 kA/m and frequency f = 300 kHz. The shaded region demarcates the clinically acceptable dose region, as determined from both the material loading capacities of the local tissue, viz. 40 mgFe/mltissue, and of the body as a whole, viz. 850 mgFe. The latter value is derived, as per EquationEquation (6)
(6) , assuming a “good” agent formulation scenario of relatively high retention (
= 0.85) and systemic tolerance (
= 0.85). The points A and B denote two possible treatment scenarios, as discussed in the text.
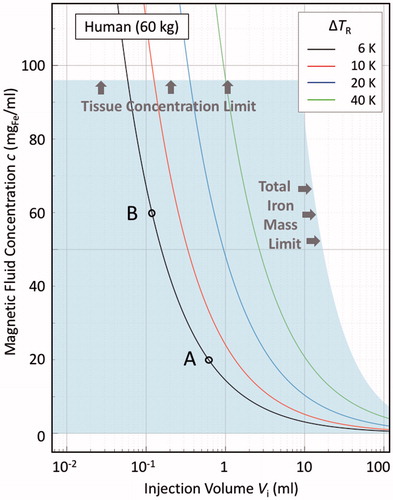
Figure 3. Illustrative bioheat model calculations in the steady-state, zero perfusion, zero metabolic heat generation limit, for a 20 g mouse, as per EquationEquation (10)(9) , under the same material conditions as in Figure 2, viz. ILP = 3.0 nHm2/kgFe, v = Vd/Vi = 2.4, and λ2 = 0.52 W/Km. The solid lines are ΔTR = 6 K isotherms, indicating the thresholds for therapeutic hyperthermia. The shaded region demarcates the murine “acceptable dose region”, as determined from both the material loading capacities of the local tissue, viz. 40 mgFe/mltissue, and of the mouse as a whole, viz. 3.5 mgFe. The latter value is derived, as per EquationEquation (6)
(6) , assuming a “good” agent formulation scenario of relatively high retention (
= 0.85) and systemic tolerance (
= 0.85). The red line, corresponding to H = 5 kA/m and f = 300 kHz (the same conditions as in ), lies outside the acceptable dose region, and as such is inaccessible. The black line, corresponding to H = 10 kA/m and f = 1 MHz, lies within the acceptable dose region, as therefore is accessible. The points A, A′, B and B′ are discussed in the text.
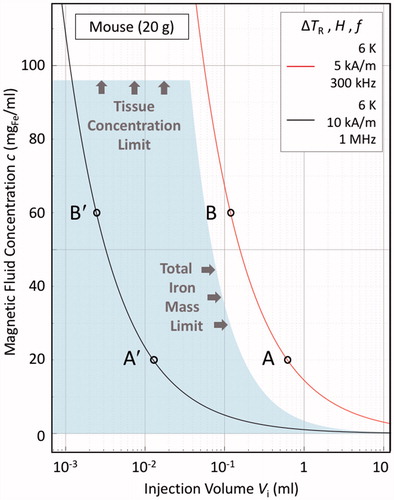
Figure 4. Comparison between maximum temperature rises ΔTmax due to magnetic heating as calculated using the first-order approximation method of EquationEquation (8)(8a) and Equation(10)
(9) , and the reported ΔTmax for a clinical study of prostate cancer patients undertaken by the MagForce team [Citation61]. The limitations of the zero-perfusion, zero-metabolism model are evident at the lower ΔTmax values, but the model is more valid as ΔTmax increases. The arrow denotes the ΔTmax value for which the temperature of the entire magnetic-nanoparticle-infused tissue volume reaches the therapeutically significant value of ΔT = 6 K or above. The dashed line is a guide to the eye only.
![Figure 4. Comparison between maximum temperature rises ΔTmax due to magnetic heating as calculated using the first-order approximation method of EquationEquation (8)(8a) and Equation(10)(9) , and the reported ΔTmax for a clinical study of prostate cancer patients undertaken by the MagForce team [Citation61]. The limitations of the zero-perfusion, zero-metabolism model are evident at the lower ΔTmax values, but the model is more valid as ΔTmax increases. The arrow denotes the ΔTmax value for which the temperature of the entire magnetic-nanoparticle-infused tissue volume reaches the therapeutically significant value of ΔT = 6 K or above. The dashed line is a guide to the eye only.](/cms/asset/bdfad95d-58a5-4ac2-bde7-56e907483789/ihyt_a_1365953_f0004_b.jpg)
