Figures & data
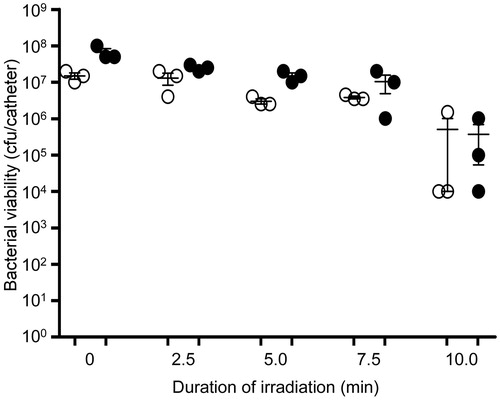
Figure 1. Structural characteristics of PDA-coated AuNCs (AuNC@PDA). TEM image of AuNC@PDA showing relative size of AuNC core and PDA coating (left). UV−Vis spectrum of AuNC@PDA in aqueous suspension (right).
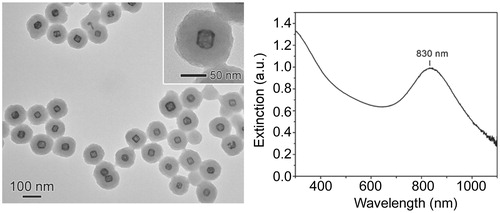
Figure 2. Temperature profile as a function of laser irradiation. Temperature was recorded in samples consisting of a colonised catheter in 500 μL of a 0.4 nM AuNC@Dap/PDA-aSpa suspension in BFM. Temperature was recorded in the absence of irradiation (time 0) and after continuous irradiation for 10 min, at which point laser irradiation was stopped and the temperature recorded for an additional 10 min. The experiment was done in triplicate with the results shown as the average ± the standard error of the mean (SEM).
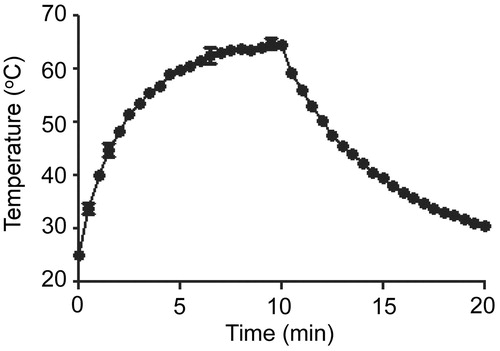
Figure 3. Killing of biofilm-associated S. aureus with AuNC@Dap/PDA-aSpa. Biofilms were allowed to form on catheters before being placed into 500 μl of biofilm medium (BFM) containing AuNC@Dap/PDA-aSpa at a final concentration of 0.4 nM. Control catheters were not irradiated (0 min), while test catheters were irradiated for the indicated period of time. The number of viable bacterial cells was then determined at 0 h (open circles, PT effect) and after an additional 24 h incubation (filled circles, antibiotic effect).
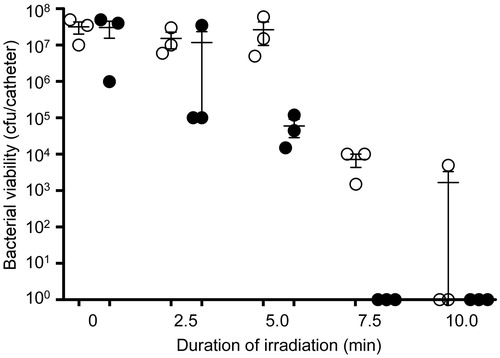
Figure 4. Antibiotic release from AuNC@Dap/PDA-aSpa as a function of laser irradiation. A 200 µl suspension of AuNC@Dap/PDA-aSpa constructs at a concentration of 0.4 nM were either not irradiated (0 min) or exposed to laser irradiation for the indicated period of time. Numbers above each bar indicate the concentration (µg/ml) observed with each sample as determined by UPLC. Concentration observed in the absence of laser irradiation (0 min) indicate dark release. Concentrations observed after irradiation indicate PT release.
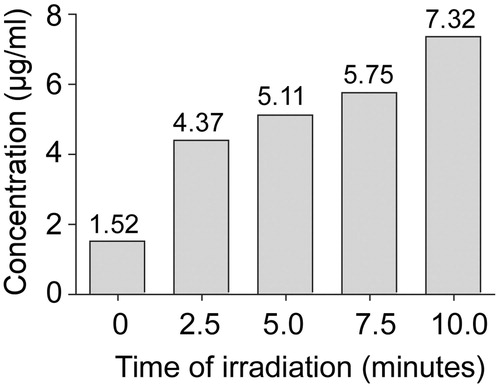
Figure 5. Temperature profile as a function of AuNC@PDA formulation. Temperature was recorded in samples consisting of a colonised catheter in 500 μL of a 0.4 nM suspension of the indicated AuNC formulation in BFM. Temperature was recorded in the absence of irradiation (0 min) and after irradiation, with readings taken at 2.5 min intervals through 10 min. The experiment was repeated 6 times with each AuNC formulation. Results are shown as the average ± the standard error of the mean (SEM).
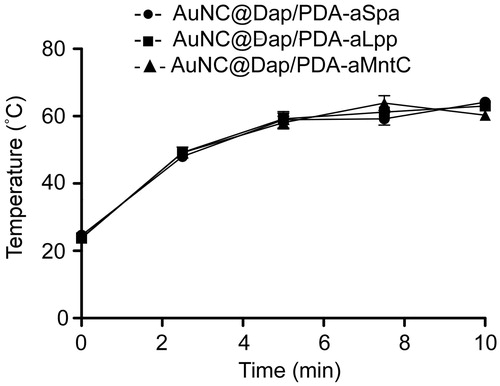
Figure 6. Localization of AuNC@PDA as a function of antibody conjugation. Images illustrate fluoresence (sGFP) and photothermal microscopy (PTM) images of S. aureus cells (green) incubated with AuNC@PDA (blue) with and without conjugation to aMntC, aLpp, or aSpa. Scale bars =10 µm.
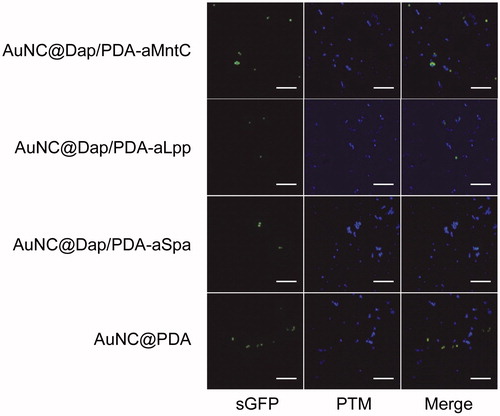
Figure 7. Killing of biofilm-associated S. aureus with AuNC@Dap/PDA-aLpp. Biofilms were allowed to form on catheters before being placed into 500 μl of BFM containing 0.4 nM AuNC@Dap/PDA-aLpp. Control catheters were not irradiated (0 min), while test catheters were irradiated for the indicated period of time. The number of viable bacterial cells was then determined at 0 h (open circles, PT effect) and after an additional 24 h incubation (filled circles, antibiotic effect).
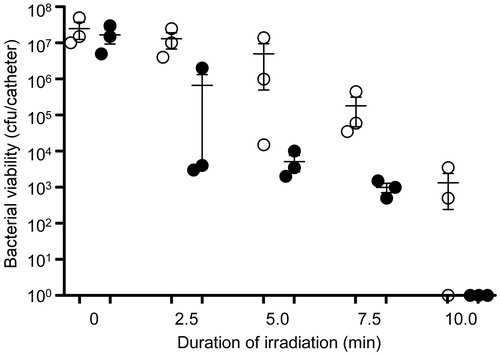
Figure 8. Killing of biofilm-associated S. aureus with AuNC@Dap/PDA-aMntC. Biofilms were allowed to form on catheters before being placed into 500 μl of BFM containing 0.4 nM AuNC@Dap/PDA-aMntC. Control catheters were not irradiated (0 min), while test catheters were irradiated for the indicated period of time. The number of viable bacterial cells was then determined at 0 h (open circles, PT effect) and after an additional 24 h incubation (filled circles, antibiotic effect).
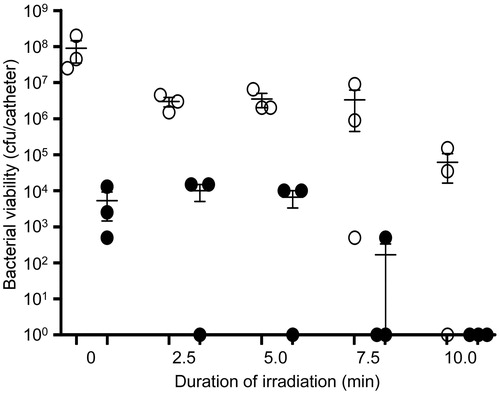
Figure 9. Antibiotic release as a function of laser irradiation. The amount of antibiotic released from the indicated AuNC@PDA formulations in the absence of laser irradiation (dark release) and after 10 min of laser irradiation (photothermal release) is shown for each AuNC formulation. Numbers above each bar indicate the concentration of antibiotic (µg/ml) observed with each formulation as determined by UPLC. Designations on the X-axis indicate the antibiotic and antibody incorporated into each formulation. Dap, daptomycin; Cef, ceftaroline; Van, vancomycin; Gen, gentamicin.
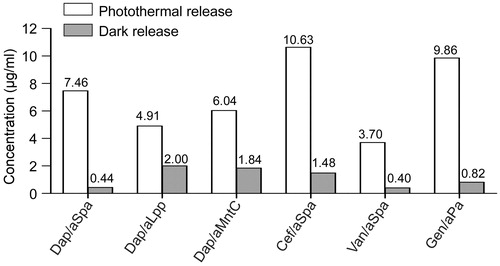
Figure 10. Comparison of antibiotic structures. Molecular structure and relative molecular weight of each antibiotic incorporated into our AuNC@PDA nanoconstructs is shown for comparison.
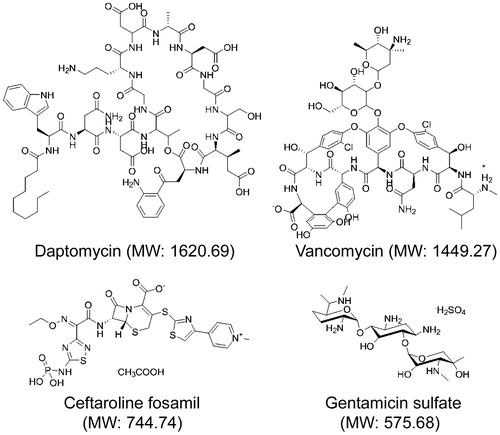
Figure 11. Killing of biofilm-associated S. aureus with AuNC@Cef/PDA-aSpa. Biofilms were allowed to form on catheters before being placed into 500 µl of BFM containing 0.4 nM AuNC@Cef/PDA-aSpa. Control catheters were not irradiated (0 min), while test catheters were irradiated for the indicated period of time. The number of viable bacterial cells was then determined at 0 h (open circles, PT effect) and after an additional 24 h incubation (filled circles, antibiotic effect).
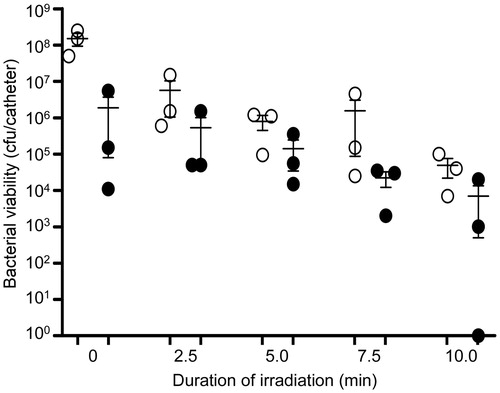
Figure 12. Killing of biofilm-associated S. aureus with AuNC@Van/PDA-aSpa. Biofilms were allowed to form on catheters before being placed into 500 µl of BFM containing 0.4 nM AuNC@Van/PDA-aSpa. Control catheters were not irradiated (0 min), while test catheters were irradiated for the indicated period of time. The number of viable bacterial cells was then determined at 0 h (open circles, PT effect) and after an additional 24 h incubation (filled circles, antibiotic effect).
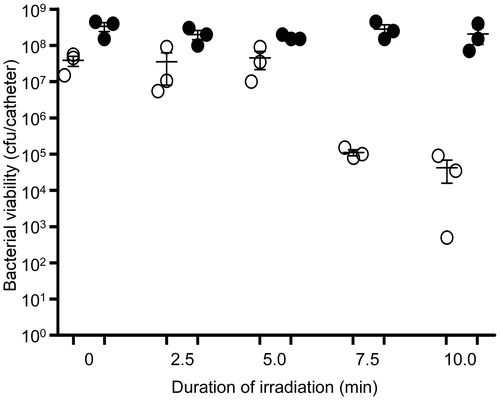
Figure 13. Killing of biofilm-associated P. aeruginosa with AuNC@Gen/PDA-aPa. Biofilms were allowed to form on catheters before being placed into 500 µl of BFM containing 0.4 nM AuNC@Gen/PDA-aPa. Control catheters were not irradiated (0 min), while test catheters were irradiated for the indicated period of time. The number of viable bacterial cells was then determined at 0 h (open circles, PT effect) and after an additional 24 h incubation (filled circles, antibiotic effect).
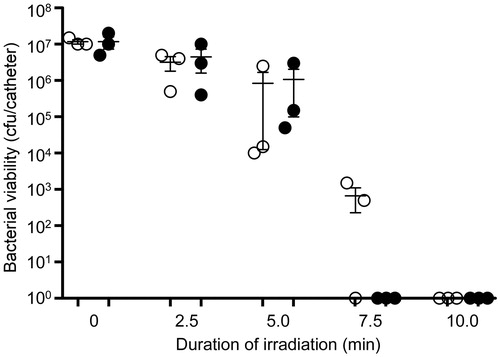
Figure 14. Killing of biofilm-associated S. aureus with AuNC@Gen/PDA-aPa. S. aureus biofilms were allowed to form on catheters before being placed into 500 μl of BFM containing 0.4 nM AuNC@Gen/PDA-aPa. Control catheters were not irradiated (0 min), while test catheters were irradiated for the indicated period of time. The number of viable bacterial cells was then determined at 0 h (open circles, PT effect) and after an additional 24 h incubation (filled circles, antibiotic effect).
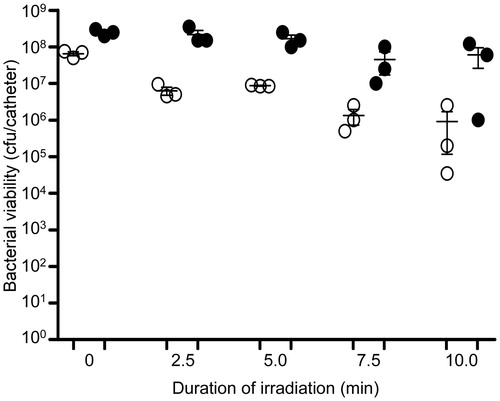
Figure 15. Killing of biofilm-associated P. aeruginosa with AuNC@Dap/PDA-aSpa. P. aeruginosa biofilms were allowed to form on catheters before being placed into 500 μl of BFM containing 0.4 nM AuNC@Dap/PDA-aSpa. Control catheters were not irradiated (0 min), while test catheters were irradiated for the indicated period of time. The number of viable bacterial cells was then determined at 0 h (open circles, PT effect) and after an additional 24 h incubation (filled circles, antibiotic effect).